Reports: DNI153543-DNI1: Concise and Logical Bottom-Up Synthetic Approach to Structurally and Electronically Designed Graphene Nanoribbons
Wesley A. Chalifoux, PhD, University of Nevada, Reno
The overarching theme of this project is the design of a concise bottom-up synthesis of narrow graphene nanoribbon (GNR) oligomers and polymers to study their structure function relationships. The initial stages of this project involved the synthesis of small molecule model systems (pyrenes and peropyrenes) to test the validity of our proposed alkyne benzannulation strategy and its applicability on larger systems (GNRs).
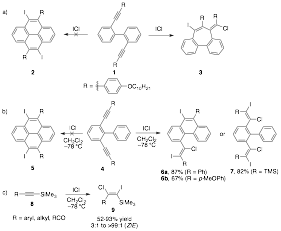
Pyrene is a small subunit of GNRs that we used as a model system for the synthesis of GNRs. Our original approach was the synthesis of diyne precursor 1 that would be later cyclized using either Lewis or Bronsted acid catalysis to generate our pyrene product 2 (Scheme 1a). Upon treatment of 1 with ICl, we did not observe the formation of pyrene 2 but believe a novel alkyne-alkyne dimerization occurred to provide product 3. We are working to repeat this interesting transformation and fully characterize product 3. We also screened a number of Lewis and Bronsted acids but were unable to generate pyrene products in good yield. We turned to a different substitution pattern, such as in compound 4, to avoid intramolecular alkyne-alkyne reactions (Scheme 1b). Treatment of this with ICl also failed to proved pyrene 5, but instead provided product 6. When compound 4 (R = trimethylsilyl (TMS)) was subjected to ICl, we exclusively formed an interesting bis-(Z)-dihaloalkene product 7. Other TMS-alkyne substrates 8 also provided (Z)-dihaloalkene products 9 in good yield as well as excellent regio- and diastereoselectivity (Scheme 1c). This reaction overrides the expected anti addition of halogen reagents to provide tetrasubstituted (Z)-1,2-dihaloalkene products via a syn addition. The products 9 contain three unique coupling sites (Cl, I, and TMS) for further chemical elaboration. This work was done exclusively by an undergraduate student, which resulted in publication.
Scheme 1. a) Attempted benzannulation of 1 with ICl resulting in the exclusive formation of product 3. b) Addition of ICl to 4 to provide monocyclized product 6 or 7 (R = TMS). c) Syn halogenation of TMS-alkyne 8 to generate tetrasubstituted (Z)-1,2-dihaloalkene products 9.
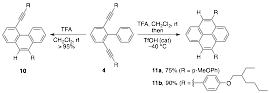
We turned our attention to Bronsted acid catalysis to invoke a double cyclization to afford pyrene products. The reaction of electron-rich diynes 4 in the presence of excess trifluoroacetic acid at room temperature resulted in the rapid formation of monocyclized product 10 (Scheme 2). Heating to reflux resulted in the slow formation of pyrene 11 over 2 days but yields suffered. We found that adding a catalytic amount of triflic acid at –40 C resulted in the clean formation of pyrene 11. We are now looking at the scope of this benzannulation methodology. It should be noted that most of this work was done by undergraduate researchers. There have been a total of three undergraduate researchers that have contributed on the synthesis of pyrenes as model systems for alkyne benzannulation. This research has been part of two senior thesis projects and presented as a poster at a recent ACS conference.
Scheme 2. Double benzannulation of 2,6-dialkynylbiphenyl substrates 4 using Bronsted acid catalysis to generate pyrenes 11.
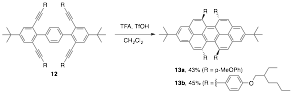
We tested our alkyne benzannulation methodology on larger systems that contain more alkynes. Treatment of 12 with TFA and catalytic TfOH resulted in the formation of tetracyclized product 13 in modest yields. We were surprised that this reaction worked as each bay region of 13 is doubly substituted and thus would have to adopt a severely twisted structure, as seen in a model compound calculated using MMFF94 (Figure 1). This methodology appears to be useful for the synthesis of other highly strained polycyclic aromatic hydrocarbons (PAHs) and we are exploring that chemistry now.
Scheme 3. Bronsted acid catalyzed tetracyclization of 12 to form twisted chiral peropyrenes 13.
Figure 1. Twisted chiral peropyrene calculated using MMFF94.
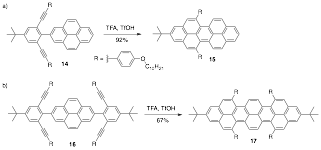
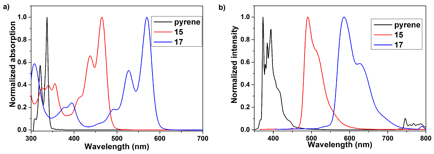
We were successful in extending this alkyne benzannulation onto pyrene moieties. Double cyclization of 14 results in high yield of peropyrene 15. We can also doubly substitute pyrene to form 16. Tetracyclization of substrate 16 resulted in moderate yield of teropyrene product 17. We see an expected red shift in the absorbance and fluorescence as a function of length in going from pyrene to peropyrene to teropyrene (Figure 2).
Scheme 4. Cyclization of pyrene substrates 14 and 16 to generate peropyrene 15 and teropyrene 17, respectively.
Figure 2. UV-vis and fluorescence spectra of pyrene, peropyrene 15, and teropyrene 17.
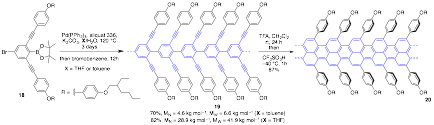
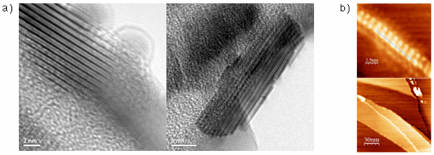
Our ultimate goal was to invoke a global alkyne benzannulation of polyalkynyl-poly-p-phenylene to generate GNRs. We indeed could synthesize monomer 18 that we subjected to Suzuki crosscoupling polymerization to provide 19 in good yield (Scheme 5). The choice of reaction solvent (toluene vs THF) makes a tremendous difference on the molecular weights obtained after polymerization, where higher molecular weights are achieved using THF. Conversion of 19 to GNR 20 was successful using TFA at room temperature for 24 h followed by the addition of a catalytic amount of TfOH at –40 C. The GNR product 20 was characterized by standard spectroscopic methods (NMR, IR, Raman, UV-vis) and by microscopy (Figure 3). The synthesis of peropyrenes, teropyrenes, and the GNR synthesis was primarily undertaken by a postdoctoral researcher who worked along with, and mentored, undergraduate researchers.
Scheme 5. Suzuki polymerization of 18 to produce 19 followed by Bronsted acid catalyzed benzannulation to generate GNR 20.
Figure 3. Characterization of GNR 20 by transmission electron microscopy (TEM) and scanning tunneling microscopy (STM).
In summary, with the assistance of the ACS-PRF fund we have collected valuable preliminary data for the rational design and synthesis of small PAHs as well as GNRs using a non-oxidative alkyne benzannulation strategy. This grant has provided a number of undergraduates the opportunity to experience research and has also provided a postdoctoral researcher valuable training for their independent career. This grant has also allowed the PI to establish a novel area of research for the synthesis of GNRs and other PAHs that has led to a number of collaborations.