Reports: UNI152945-UNI1: Development of Copper(I) Catalysts for Photoredox Catalysis
Katrina H. Jensen, PhD, Black Hills State University
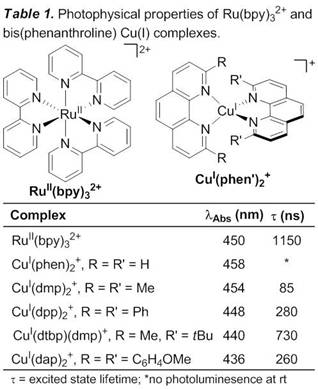
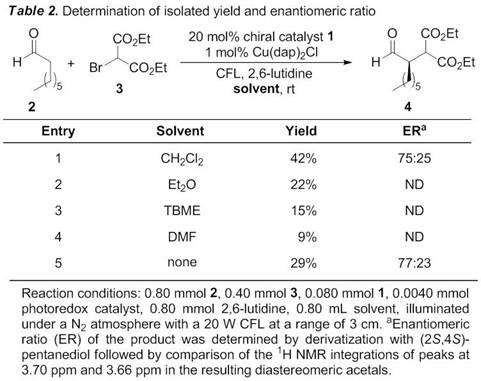
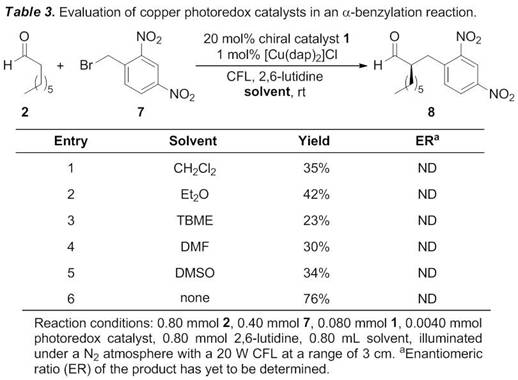
Katrina H. Jensen, PhD, Black Hills State University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society