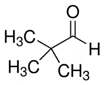
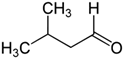
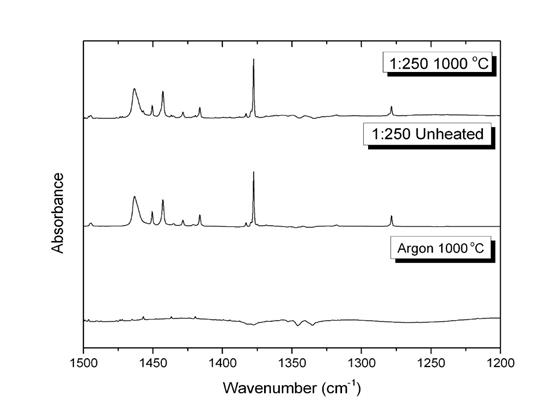
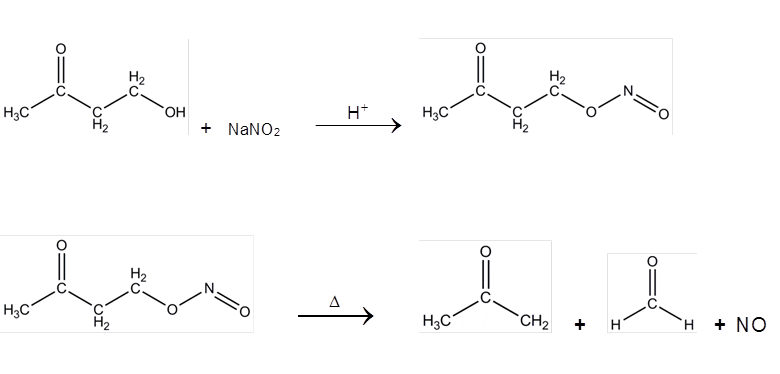
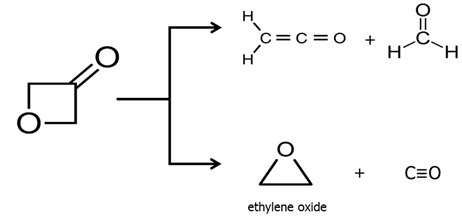
Reports: UR453036-UR4: FTIR Analysis of the Radical and Molecular Products of Thermal Decomposition of Aldehydes and Nitrite Esters
Laura R. McCunn, PhD, Marshall University
1.
Wright, E. M.; Warner, B. J.; Foreman, H. E.; McCunn, L. R.; Urness, K. N.
Pyrolysis Reactions of 3-Oxetanone. J. Phys. Chem. A 2015, 119,
7966-7972.