Reports: DNI754462-DNI7: Fundamental Studies of Material Properties of Chemically Cross-Linked Gels Concentrated above the Overlap Concentration
Kelly Schultz, PhD, Lehigh University
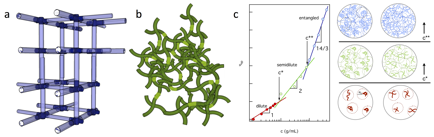
This work is motivated by the need for manipulation of polymeric cross-linked gel properties to address unique situations faced on-site during enhanced oil recovery (EOR). To understand the function of these gels, we must first understand how precursor solution interactions change material properties during gelation. Schematics of gel structures below, showing an ideal structure, and above c*, showing the interplay between entanglements and cross-linking, are illustrated in Figure 1a and b. Polymeric solutions have three regimes in the concentration-viscosity curve, the dilute, semi-dilute and entangled, illustrated in Figure 1c both in the viscosity curve on the left and illustrations on the right for linear and multi-arm polymers. The goal of this work is to establish a fundamental understanding of how the interplay between entanglement and cross-linking changes the polymeric assembly, final structure and properties and overall stability of the network using rheological characterization.
Figure 1. Illustrations of cross-linked gel structures (a) below and (b) above the overlap concentration. (c) Concentration-viscosity curve (adapted from Colby and Rubinstein, Polymer Physics, 2003) with schematics of polymer solutions in the concentration regimes.
Measuring the overlap concentration of polymeric precursor solutions with passive microrheology
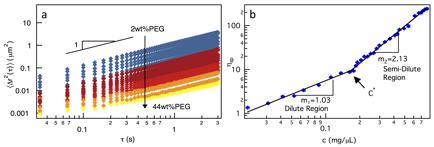
The scaffold materials that we are interested in are poly(ethylene glycol) (PEG) based scaffolds. This polymer is chosen due to its wide applicability across many fields and availability in various sizes and configurations. In particular, we have focused on the 4-arm star configurations (Mn20 000 g/mol). Multiple particle tracking microrheology (MPT) is used to measure the viscosity of PEG solutions over a wide range of concentrations, identifying the value of c*. In MPT, fluorescently labeled probe particles are embedded into the material and video microscopy is used to capture their movement. The movement of these particles is tracked and the rheological properties are calculated using the Generalized Stokes-Einstein Relation. Using MPT, we can measure more samples, due to the small amount of material required and speed of data acquisition. The results of MPT measurements are shown in Figure 2, where the mean-squared displacement shows the decrease in particle diffusivity (Figure 2a) and the overlap concentration is 14.3±3.6 wt% (Figure 2b).
Figure 2. MPT of 4-arm star PEG molecules. The measured (a) mean-squared displacements and (b) viscosity identifying the overlap concentration.
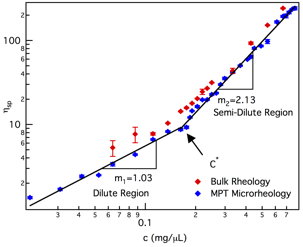
Validation of overlap concentration using bulk rheology and theoretical calculations
Bulk rheology measures the viscosity of polymer solutions and confirms the value of c* identified with MPT; Figure 3. Bulk rheological measurements agree with MPT measurements and the value of c* identified is within error of the value measured with MPT, 16.0±7.0 wt% (bulk) and 14.3±3.6 wt% (MPT). Both of these values are in agreement with the overlap concentration calculated for star-polymers using the equation
where M is the molecular weight, Rgstar is the radius of gyration of the star polymer defined as ![]() where Rgarm is the radius of gyration of a polymer arm, f is the number of arms and NAis Avogadro's number. The calculated value of c* is 15.6 wt%.
where Rgarm is the radius of gyration of a polymer arm, f is the number of arms and NAis Avogadro's number. The calculated value of c* is 15.6 wt%.
Figure 3. Measurements of the overlap concentration using MPT and bulk rheology
Developing techniques to measure equilibrated properties during photopolymerizationIn this work, we stop photopolymerization and measure material properties at different extents of reaction. A low powered UV lamp held at a constant distance from the sample is used to expose samples for a few seconds. This experimental setup was used to measure a chain-growth polymerization reaction between a 4-arm star PEG-acrylate and PEG-dithiol. In this experiment, we obtain reproducible measurements of gelation as the extent of reaction is varied.
Measuring gelation below the overlap concretion with microrheology
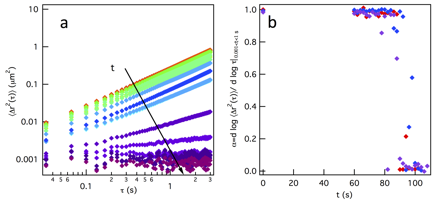
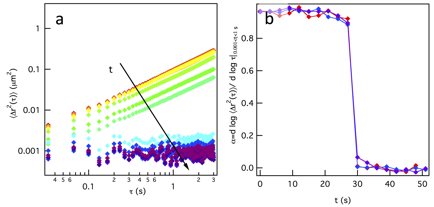
Gelation is measured for a gel composition of 3 wt% PEG-acrylate and equal reaction groups of PEG-dithiol. Data shows that the gelation reaction is successfully stopped at different extents of reaction. Figure 4a, shows the mean-squared displacements (MSD, ![]() ) measured using MPT. The decrease in the slope of the MSD,
) measured using MPT. The decrease in the slope of the MSD, ![]() , from 1 to approximately 0, indicates that the material is originally a liquid, it goes through a sol-gel transition and then becomes a stiff gel. Gelation occurs quickly once polymer clusters have grown and that can be seen in Figure 4b, where α=1 and a fast transition occurs to α=0.
, from 1 to approximately 0, indicates that the material is originally a liquid, it goes through a sol-gel transition and then becomes a stiff gel. Gelation occurs quickly once polymer clusters have grown and that can be seen in Figure 4b, where α=1 and a fast transition occurs to α=0.
Figure 4. (a) MSD and (b) slopes of 3 wt% PEG-acrylate gel.
Measuring gelation at and above the overlap concentration with microrheology
The gelation reaction of PEG-acrylate and PEG-dithiol is measured at the overlap concentration, 16 wt% PEG-acrylate and equal reaction groups of PEG-dithiol. As expected, increasing the polymer concentration results in faster gelation and sol-gel transition, Figure 5a and b. Current work is focusing on measuring the gelation reaction above c*, 25 wt% PEG-acrylate and equal reaction groups of PEG-dithiol. We expect to measure a greater contribution of polymer interactions at 25 wt%.
Figure 5(a) MSD and (b) slopes of 16 wt% PEG-acrylate gel.
Identification of material critical values during the gelation reaction
The sol-gel transition is determined using time-cure superposition (TCS). TCS identifies the critical relaxation exponent (n), a measure of how much energy a network stores, and the critical gelation time (tc), time the first sample spanning cluster appears. For 3 wt% and 16 wt% PEG-acrylate n = 0.69±0.09 and tc~90 s and n = 0.61±0.10 and tc~30 s, respectively. This indicates that the material stores more energy than it dissipates. This analysis confirms that the gelled structure is not changing below and at c*. We expect a larger variation in structure and gelation with measurements well above c*.
Impact
Fundamental understanding of how polymer interactions in concentrated solutions change the structure and properties of gels and development of accessible measurement techniques will enhance the applications of gels in the chemical and petroleum industry. Support from this ACS-PRF DNI has resulted in development of techniques and materials characterization that is central to the PI's research program. This work provides essential preliminary data that will contribute to proposals that expand in scope. This grant has supported two doctoral students and provided the foundation and resources for related research by two undergraduate students.