Reports: ND252964-ND2: 238U/235U Ratio as a Tracer of Paleoredox Conditions: Application to the Oxygenation of the Global Ocean Throughout Earth History
Nicolas Dauphas, PhD, University of Chicago
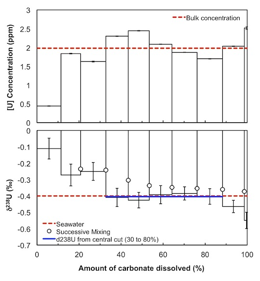
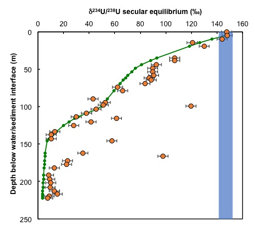
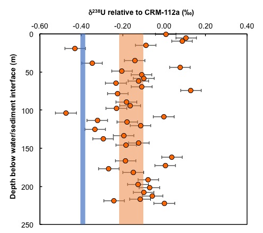
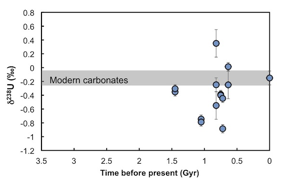
Nicolas Dauphas, PhD, University of Chicago




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society