Reports: ND153765-ND1: Gold-Catalyzed Photoredox Transformations
Louis Barriault, University of Ottawa
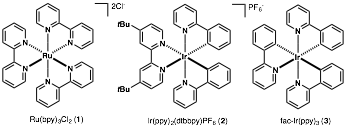
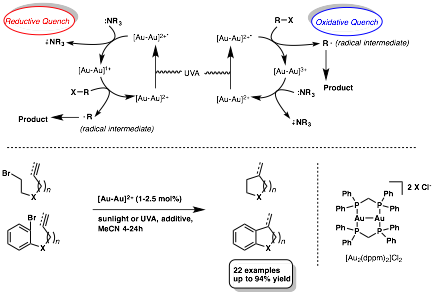

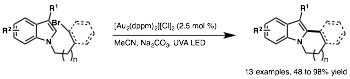
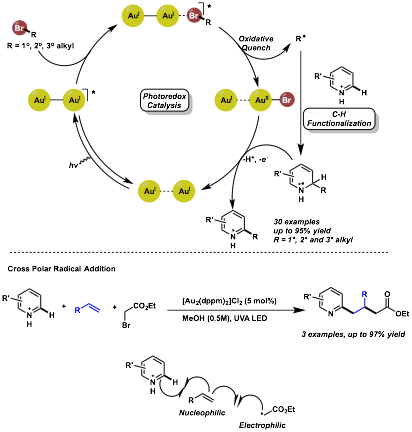
Louis Barriault, University of Ottawa



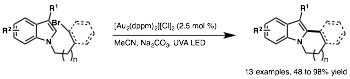

Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society