Reports: DNI354225-DNI3: Fundamental Studies toward the Copolymerization of Ethylene with Carbon Dioxide: Bimetallic Group 10/Main Group Catalyst Synthesis and Reactivity
Ian A. Tonks, PhD, University of Minnesota
Research in the Tonks lab is focused on synthesizing bimetallic catalyst systems to copolymerize carbon dioxide with ethylene into biodegradable polyesters. CO2/C2H4 copolymerization is thermodynamically disfavored below a 1 : 2.37 ratio of CO2 : C2H4. As a result, hypothetical CO2/C2H4 copolymerizations with late transition metal catalysts proceeding through consecutive insertions of CO2 and C2H4 are kinetically inaccessible because there is a high-energy barrier en route to endothermic CO2 insertion relative to essentially barrierless exothermic C2H4 insertion.
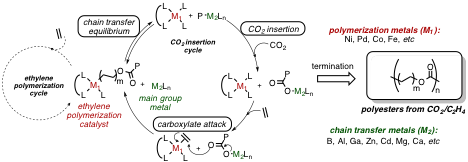
Figure 1. Bimetallic chain transfer strategy for CO2 and ethylene copolymerization.
Our approach has been to utilize recent advances in chain transfer polymerization to in situ functionalize growing metal-polyethyl chains with CO2 (Figure 1). This will be accomplished by designing polymerization systems capable of polymeryl chain transfer equilibrium between two different metal centers: one transition metal center that is capable of ethylene polymerization, and a second metal center uniquely capable of alkyl (polymeryl) group insertion into CO2 to form a carboxylate. The resulting polymeryl carboxylates can then rebound to the ethylene polymerization metal via Wacker-type ethylene carboxylation, closing the catalytic cycle.
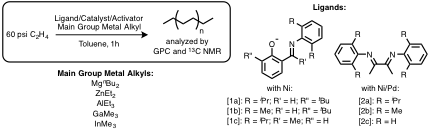
Figure 2. Group 10 catalyst systems investigated for polymeryl chain transfer. Catalysts [2x]Ni were found to effectively chain transfer with ZnR2.
Late transition metal polymeryl chain transfer. Prior to the start of this grant period, we systematically studied the potential for chain transfer between group 10 polymerization catalysts and main group elements (Figure 2). The catalyst systems with the most efficient chain transfer were found to be cationic (α-diimine)Ni catalysts paired with dialkyl zinc chain transfer reagents. In these systems, chain transfer was found to be dependent on the sterics of both the catalyst and the chain transfer reagent (CTR). When less sterically encumbered catalysts or CTRs were utilized, the relative rate of bimetallic chain transfer to chain propagation was increased; however, in cases where chain termination via β-H elimination was extremely rapid, chain transfer to Zn was kinetically unviable. Chain transfer from (α-diimine)Ni catalysts to Zn alkyls is also very sensitive to the strength of the Zn-C bond: ZnMe2 (186 kJ/mol) is a significantly poorer chain transfer reagent than ZnEt2 (157 kJ/mol).
Synthesis of homo- and heterobimetallic complexes. Our initial results indicated that the rates for intramolecular chain transfer from Ni catalysts are too slow to be kinetically viable in a potential CO2/ethylene polymerization cycle. In order to increase the rates, we have focused our research efforts on synthesizing bridging, bimetallic late transition metal/main group metal complexes based on ligand frameworks that have been successful for olefin polymerization.
First, we have synthesized various mono- and bimetallic Ni complexes based on β-oxo-δ-diiminate ligand frameworks (Figure 3). The monometallic complexes all share an interesting feature: the potential for enamine/imine tautomerization on the "free" arm of the ligand set; under certain reaction conditions we are able to isolate either tautomer. In conjunction with the deprotonated Li/Na/K series, the prototropism of this ligand will allow us to study the effect of bridging hydrogens and metals on olefin polymerization catalysis. Initial polymerization results indicate that the tolyl-Ni catalysts listed in Figure 3 are competent for ethylene polymerization, yielding activities on the order of 1x104 g/mol/hr. Interestingly, they are also capable of polymerization in the presence of Lewis basic additives such as NEt3, indicating that secondary metal effects may play a role in promoting the polymerization of polar comonomers. Our ongoing research with this ligand set involves further polymerization studies, and synthetic studies to synthesize heterobimetallics involving Zn, Mg, and transition metals paired with Ni.
Figure 3. Tautomeric and bimetallic β-oxo-δ-diiminate Ni complexes are all capable of ethylene polymerization in the presence of Lewis bases.
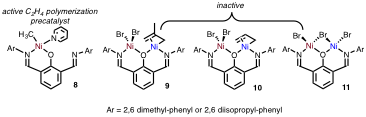
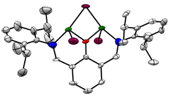
Figure 4. Homobimetallic Ni complexes based on the 2,6-diiminophenolate ligand set.
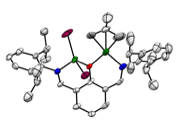
Second, we have also synthesized related complexes based on 2,6-diiminophenolate ligands. By utilizing this ligand set, we are capable of synthesizing several Ni homobimetallic complexes (Figure 4) in addition to myriad mononickel complexes. It is interesting to note that in these homobimetallics, the Ni metal centers are skewed from the phenol plane, indicating severe steric strain in the system (Figure 5). Despite this strain, ligands are capable of bridging between the two Ni centers, which serves as a structural model for our proposed chain transfer process. Unfortunately, of all of the complexes synthesized with this ligand only the NiMe(py) complex 8 polymerizes ethylene, and attempts to synthesize bimetallics based on this extremely reactive monometallic complex have been unsuccessful. We are currently exploring alternative synthetic strategies to access bimetallics possessing Ni-Me groups.
Figure 5. X-ray crystal structures of 9 (left) and 11 (right) showing skewed Ni-Ni geometries.
Finally, we have synthesized Ni complexes of a salicylaldimine ligand containing a bipyridine pendant. Much like the Ni phenolate complexes, these complexes are generally incapable of ethylene polymerization in the absence of an activator. However, when ZnCl2 is added to the Ni tolyl pyridine complex 12 or 13, high ethylene polymerization activity is observed. We are currently investigating the mechanism by which ZnCl2 promotes polymerization: it could occur via simple pyridine sequestration by the Lewis acid, or via catalyst modification by ZnCl2 coordination to the open bipyridine pocket.
Figure 6. Addition of ZnCl2 has a profound effect on ethylene polymerization with Ni salicylaldimino(bipyridine) catalysts.
Impact on Career and Students. The research performed under the ACS PRF grant window has had a profound effect on my career and my students' careers. Two students (Dr. Ryan Hue and Mr. Jimmy Chiu) have presented this research at the ACS National Conference in Denver, giving them firsthand experience presenting research projects to a broad audience. Their presentations attracted the attention of the research arm of Bridgestone-Firestone, and as a direct result of their research and presentations, we are now in the process of setting up a new collaboration with Bridgestone. The early results that have been obtained under PRF funding have also provided the initial directions for two thesis projects for 3rd year students in the research group (Mr. Jimmy Chiu and Ms. Abigail Smith).