Reports: DNI1052906-DNI10: Dynamic Synthesis and Assembly of Nanoporous Materials
Ronald A. Smaldone, PhD, University of Texas at Dallas
ACS-PRF Grant Impact on the Smaldone Group – Over the past two and a half years at the University of Texas at Dallas, our group has thrived through the generous support of the ACS Petroleum Research Fund. During my first summer at UTD, I was able to support two graduate students (Fei Li, Arosha Karunathilake) on research assistantships that otherwise would have required us to use valuable startup funds. With their first summer available for a complete focus on research, we were able to generate many of the results in this report. Fei Li and Dr. Christina Thompson a postdoctoral research associate partially supported by this grant, were able to synthesize and evaluate the hexaphenylbenzene (HEX) and hexabenzocoronene (HBC) derivatives that are described below and resulted in our first publication in Chemical Communications and a follow up full paper in Macromolecules. Most recently, we have been synthesizing a series of HEX-based covalent organic frameworks (COFs) and porous polymers based on reversible dynamic bond formation. In addition to research support, the ACS-PRF allowed me to send all of my students to the ACS National Meeting in Dallas, TX. This was an invaluable opportunity to engage with the chemical community far earlier in their careers than many graduate students do. Over the last year this grant has supported one graduate student, and will continue to do so on an extension granted by the ACS-PRF.
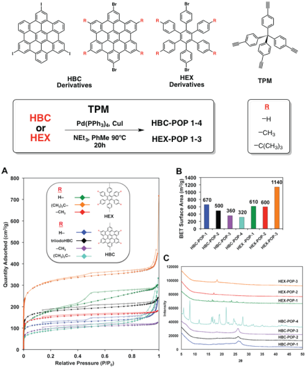
Figure 1. (top) TPM-based HEX and HBC-POPs synthesized by our lab. (A) N2 isotherms collected at 77 K for each POP and (B) the BET surface areas obtained from the isotherms. (C) Powder X-ray diffraction patterns collected for each POP.
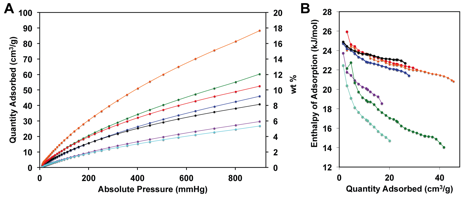
Results from ACS-PRF Supported Research – To date, our laboratory has synthesized and characterized a series of porous organic polymers (POPs) based on HBC, HEX and tetraphenylmethane (TPM) monomers. One of the main goals of this work was to expand on our previous report on HBC-based POPs by studying the effects that polycyclic aromatic hydrocarbons (PAH), and rim functional groups exert on the properties of the POPs. Figure 1 describes the synthetic procedure used to obtain these copolymers designated HEX- and HBC-POPs from the corresponding aryl halide monomer and TPM. To determine the accessible surface areas and pore size distributions, these POPs were evaluated through nitrogen adsorption experiments at 77 K. The resulting isotherms and powder diffraction patterns are shown in Figure 1a-c. The BET surface areas obtained from these isotherms ranged from 315 m2/g for HBC-POP-4 to 1141 m2/g for HEX-POP-3. Carbon dioxide isotherms (Figure 2a) were collected at 273 K and 298 K, with HEX-POP-3 showing the highest storage capacity at 18 wt% - relatively high for POPs without polar functional groups. Heats of adsorption (HOA) for CO2 were calculated from these data for all polymers (Figure 2b).
Figure 2. (A) CO2 isotherms (273 K) and (B) isosteric enthalpies of adsorption for HBC and HEX-POPs.
Based on the BET surface areas alone, the HEX-based POPs generally have a higher surface area than the HBC-POPs. This can likely be attributed to several factors: 1) overall better solubility of the HEX monomers compared with the HBC monomers, resulting in a higher degree of polymerization and 2) poorer self-association between the bulkier, less ¹-conjugated HEX monomers. However, if we consider the HEX and HBC-POPs separately, the trends with regard to peripheral functional group size are opposite. For example, increasing the size of the functional groups on the edges of the HBC monomers actually serves to decrease the overall surface area and sorption properties of the polymers. We hypothesize that this is a result of the fact that the HBC-POPs tend to form small micropores that become blocked or occupied when methyl or t-butyl groups are added. HEX-POPs, on the other hand, display the opposite trend – larger, more solubilizing functional groups improve the overall porosity.
The microscopic structure of the HBC and HEX-POPs were investigated using powder X-ray diffraction methods. Interestingly, we found that the polymers have little long-range order, while they do display a strong reflection at ~26 degrees 2 theta, representing a distance (~3.5 ) that corresponds to face-to-face ¹-stacking interactions (Figure 2c). At this juncture, we seek to explore methods to utilize these interactions to control and direct the ordered formation of 2D porous polymers.
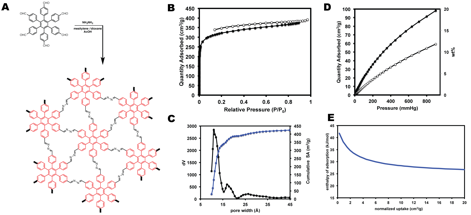
Toward Polyphenylene Porous Polymers and COFs under Dynamic Control – Based on these previous observations, strong ¹-stacking interactions have shown to be somewhat detrimental to the surface area and sorption properties of porous polymers containing HEX or HBC units. However, as a consequence of the 2D layered structures of COFs, these interactions could be beneficial to direct and stabilize COF-based materials. To explore this possibility, we have synthesized a HEX-based monomer containing aldehyde functionality that can be polymerized under dynamic conditions. Shown in Figure 3 are the results of N2 and CO2 uptake experiments. The N2 isotherm gave a BET surface area of 1100 m2/g with a major pore size peak at ~10-11 which corresponds with the expected pore size of the porous polymer depicted in Figure 3a from molecular models. Powder X-ray diffraction patterns have not shown any appreciable crystallinity within the HEX-azine polymers. Over the next year we plan to explore the effects of the choice of co-monomer on the crystallinity and resistance to solvent degradation as well as the increased ¹-stacking that can be achieved by using extended aromatic structures.
Figure 3 – (A) Synthesis of a 2D azine linked HEX-based microporous polymer. (B) N2 isotherm (77 K) for the azine linked HEX porous polymer. (C) Pore size distribution by NLDFT and cumulative surface area measurements. (D) CO2 isotherms at 273 K (filled circles) and 298 K (open circles). (E) Heat of adsorption measurement for CO2.