Reports: DNI1053638-DNI10: Bi-Compartmental Targeting Vehicles for In-Situ Catalytic Recovery of Hydrocarbon Molecules
Ning Wu, Ph. D, Colorado School of Mines
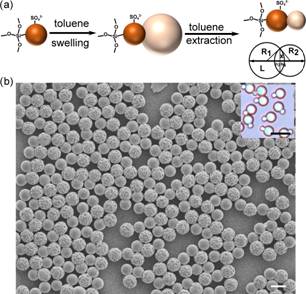
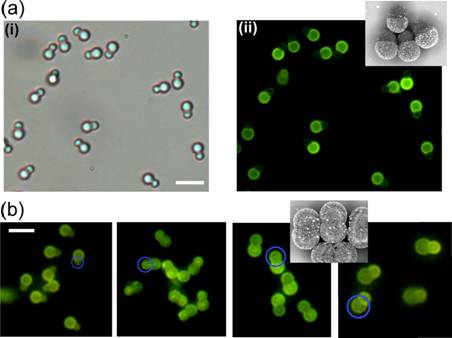

Ning Wu, Ph. D, Colorado School of Mines



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society