Reports: ND554141-ND5: Suspension Interactions via Gradients Generated in situ: Breaking Emulsions and Triggering Flocculation
Todd M. Squires, University of California (Santa Barbara)
It has been known that particles generally migrate diffusiophoretically when suspended in a solution gradient; however, it has remained challenging even to visualize diffusiophoretic migration directly, much less to measure the broad set of diffusiophoretic mobilities of different particles in solute/solvent gradients of different concentration and magnitude that will be required for our project.
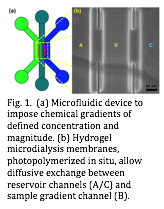
To this end, we had previously developed methods to design and impose controllable solute/solvent gradients in a microfluidic system, wherein photopolymerized hydrogel membranes act as microdialysis membranes (fig. 1).
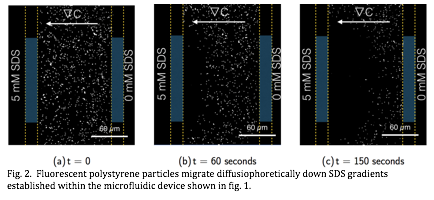
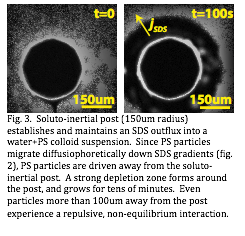
Using this system, we have measured the diffusiophoretic migration of various species, including fluorescent polystyrene particles, under gradients of the ionic surfactant sodium dodecylsulfate (SDS), as a function of concentration and gradient strength. Fig.3. In tandem, we have developed an experimental system in which a defined structure (fig. 3) establishes a long-lived outflux of SDS. In the near-term, we intend to quantitatively compare our theoretical models with experimental results, and thus to publish a compelling, proof-of-principle demonstration of this broad mechanism.