Reports: UR151819-UR1: Phosphorus-Hydrogen Activation Using Alkynylmetal Complexes: New Methodology for the Preparation of Metallopolymers
Robert A. Stockland, Bucknell University
Overview: During this year of the project, our efforts were focused on the synthesis of new metallopolymers.
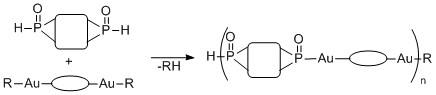
Our approach to the synthesis of the metallopolymers used protodeauration reactions between bisphosphites and organogold compounds to link the components together. During the earlier phases of the project, we focused on devising the synthetic methodology for the preparation of a range of functionalized organogold precursors containing various tethers between the two gold centers. A butanediol derived bisphosphite was used as the second component of the condensation polymerization. The metallopolymers were successfully generated under bulk conditions as well as in solution.
Scheme 1. General description of our approach to the synthesis of metallopolymers.
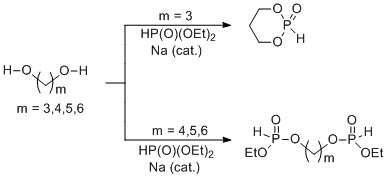
To expand the scope of the chemistry, we generated several additional bisphosphites and used them to construct new metallopolymers. Our initial studies used a butanediol derived species, and the synthesis of this bisphosphite was relatively straightforward. However, the chemistry was less successful when analogous reactions were carried out using longer or shorter chain diols. When propanediol was screened, a complex mixture of transesterification products including diethylphosphite, monofunctionalized and difunctionalized (bisphosphite), as well as a significant amount of 1,3,2-dioxaphosphorinane-2-oxide was observed. Naturally, the intramolecular transesterification was favored over the intermolecular version. This phosphite was the major component of the reaction mixture but was unusable for the polymer chemistry. Attempting to increase the amount of the bisphosphite by changing the reaction temperature, base, or reaction time were unsuccessful. Separating the bisphosphite from the remaining components of the mixture by distillation was low yielding, and purification by column chromatography was only partially successful. When pentanediol and hexanediol were screened, it was easier to remove the diethylphosphite precursor, but complex mixtures were still obtained. In these cases, purification by column chromatography was low yielding and considerable overlap between components was observed despite extensive solvent/gradient screening. Only small amounts of the cyclic monophosphites were observed in these reactions, and several higher molecular weight species were generated. Analysis of these components suggested the formation of oligomers as well as potentially interesting macrocyclic bisphosphites.
Scheme 2. Synthesis of bisphosphite monomers.
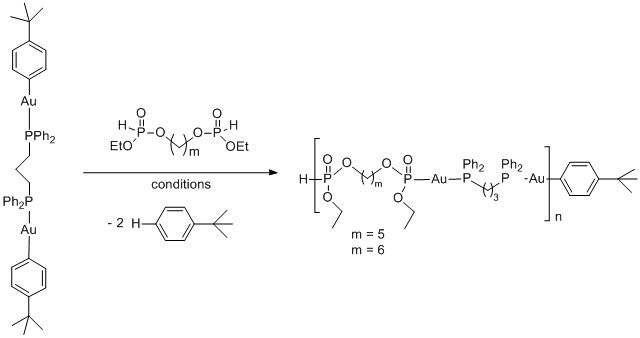
Small amounts of the bisphosphites derived from pentanediol and hexanediol were isolated and screened for activity in the polymerization reactions. For these reactions, a single gold containing precursor was used as a representative model [(dppp)Au2(C6H4Bu)2]. When the polymerization was carried out under solvent free conditions at room temperature, mixtures of oligomers and starting materials were obtained. Increasing the temperature to 50 °C, resulted in an increased consumption of starting materials as well as increased amount of the oligomer/polymer. If the polymerization was carried out in solution (THF or benzene) no reaction was observed at room temperature and the reactions needed to be heated to 60-80 °C in order to generate oligomer/polymer.
Scheme 3. Synthesis of metallopolymers through protodeauration.
During this phase of the project, I was fortunate to have a number of very talented undergraduates working on this chemistry (two were directly funded by this PRF award). Returning veterans from year 2 included Kevin Garcia (BS Chemistry '16), and Rosa Ciccarelli (BS Chemistry '16). Joining the project this year were Taylor Lord (BS Chemistry '18), Kurt Vostal (BS Chemical Engineering '15), and Wade Heidel (BS Chemical Engineering '19). Several of these students traveled to the 250th National ACS meeting in August to present posters on their work:
CHED228: Gold catalyzed addition of diphenylphosphinic acids to alkynes, Kevin Garcia, Daniel Fraccica, Kurt Vostal, and Robert A. Stockland Jr.
CHED231: Generation of metallopolymers using transesterification, Kevin Garcia, Kurt Vostal, and Robert A. Stockland Jr.
Summary: During this phase of the project, we focused on expanding our metallopolymer chemistry. Specific areas of investigation included devising a reliable set of conditions for the synthesis of several additional bisphosphite monomers, increasing the scope of our protodeauration reaction. The physical properties of the new materials will be studied during the final phase of the project.