Reports: ND153964-ND1: The Development of a New Class of Atropisomeric Heteroaromatic Biaryl Ligands for Enantioselective Transformations
Aaron Aponick, University of Florida
The original proposal entitled "The Development of a New Class of Atropisomeric Heteroaromatic Biaryl Ligands for Enantioselective Transformations" described two specific aims:
1. To prepare chiral ligands and complexes with pi-stacking components and probe structural effects. The goal of this aim is to prepare and study chiral ligands by incorporating chelating heteroatoms (P,N,O) into the basic scaffold shown above.
2. To employ chiral complexes with the stabilized biaryl motif in enantioselective reactions. The goal of this aim is to capitalize on the unique properties of heterocyclic, axially chiral biaryls for use as catalysts.
Progress towards Aim 1:
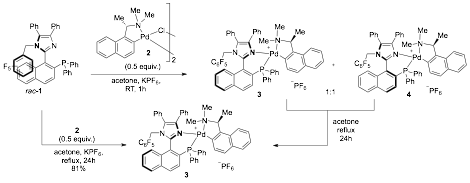
For this aim, we were able to quickly make non-racemic material (Scheme 1). The resolution of axially chiral P,N-ligands usually requires the use of a chiral palladium complex dimer such as 2. Upon complexation of the P,N-ligand 1 (now called StackPhos) with 2, a 1:1 mixture of diastereomers is typically formed and separated by fractional crystallization. After decomplexation of the chiral palladium moiety, a single enantiomer of the ligand is obtained. This method works exceedingly well over a wide range of axially chiral P,N-ligands. We performed an experiment at ambient temperature in acetone in which the racemic ligand rac-1 and the palladium dimer 2 were mixed in the presence of potassium hexafluorophosphate, and a 1:1 mixture of diastereomers 3 and 4 was observed (Scheme 1). This equimolar mixture was isolated and fractional crystallization was attempted, however, after extensively screening conditions this method proved to be unsuccessful. Interestingly, during attempts to optimize this separation process, it was realized that the ratio of diastereomers would slightly change and this was seemingly not due to a fractional crystallization. This observation was made when conditions at higher temperatures were employed and this suggested that it may be possible to interconvert the two diastereomers. Based on this data, we were encouraged to heat the diastereomeric mixture to possibly equilibrate the system to a single diastereomer, although this would rely on a significant energy difference between 3 and 4. To probe this, a 1:1 mixture of diastereomers 3/4 in acetone was heated at 60 °C for 24h. To our delight, a single diastereomer was observed as judged by 1H NMR. To evaluate the practicality of this process, the experiment was performed on a larger scale by mixing racemic phosphine 1 with palladium complex 2 and KPF6 in acetone. After refluxing the mixture for 24 hours the solution was filtered off, the solvent removed, and a single diastereomer was observed in the NMR of the crude reaction mixture. A single recrystallization gives the pure diastereomer 3 in 81% yield. As both enantiomers of 2 are available, this process allows the rapid conversion of racemic ligand to a single enantiomer of 3, without the classical problem of losing 50% of the material during a kinetic resolution process.
Scheme 1
With a good synthesis of a single diastereomer of 3 in hand, what remained was to isolate free StackPhos by decomplexation. With the demonstration of the stereochemical lability of 3, albeit at elevated temperatures, isolating StackPhos without erosion of the ee was of concern with the potential for this problem to originate either by epimerization or racemization at the complex or free ligand stages respectively. The barrier to rotation of StackPhos was initially an unknown and careful analysis of the ee of free ligand would be needed after decomplexation. Fortunately, after oxidation of rac-1 to the corresponding phosphine oxide a baseline separation was observed.
To isolate free StackPhos, decomplexation was effected by reaction of ent-3 with dppe. This enantiomer of 3 was utilized so that direct comparison to known copper complexes could be made vide infra. The initial experiments were conducted at ambient temperature in dichloromethane and 1 was recovered in high yield after column chromatography (Table 1, Entry 1). Surprisingly, the enantiomeric excess of StackPhos 1 ranged from 88 to 92% with these conditions and clearly this procedure needed improvement. Reducing the temperature improved the optical purity to 98% ee, and provided ligands to explore in Aim 2. We have also begun to prepare and isolate complexes of other metals such as iridium as well as generate catalysts in situ to explore new reactions.
Table 1
Progress towards Aim 2:
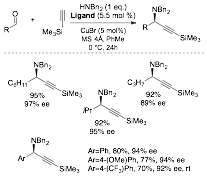
When the grant was submitted, we had some good preliminary data suggesting that the ligand would be good for the enantioselective A3-coupling. Now, with a source of non-racemic ligand in high ee, we were able to develop this method (Scheme 2).
Scheme 2.
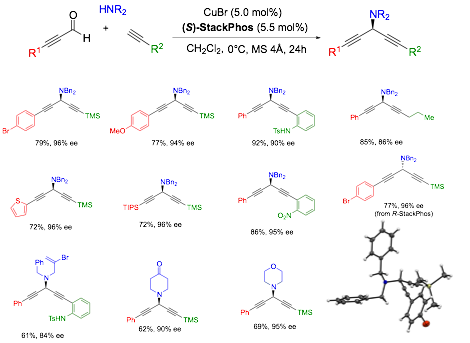
We have furthered this A3 coupling chemistry to permit the use of ynals to produce aminated skipped diynes in very high ee (Scheme 3). While there are many potential side reactions that could be envisioned, the reaction proceeds smoothly to provide the desired products in high yield and excellent ee's. The reaction is extremely versatile, allowing a wide range of compounds to be easily prepared. Additionally, since two alkynes are present, if one pair of reagents fails, the aldehyde is easily place on the other alkyne, enabling a second attempt. More importantly, if both work, this allows access to either enantiomer with a single enantiomer of StackPhos. This work will be submitted for publication shortly.
Scheme 3
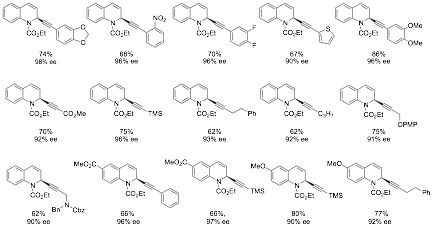
Keeping with alkyne chemistry, we have also developed an extremely efficient method for the dearomative alkyne addition to in situ generated quinolinium salts (Scheme 4). As can be seen, a wide variety of alkynes work in this chemistry including a wide range of electron rich and deficient aromatic alkynes, propionates, silyl acetylenes, propargyl nucleophiles, and even aliphatic alkynes. Furthermore, the quinoline can be substituted with electron donating and electron withdrawing substituents. All reaction proceed smoothly and provide the products in very high ee. This work has been accepted for publication and is “in press” in Angewandte Chemie.
Scheme 4
We are currently in the process of further developing the chemistry of StackPhos and have several more reactions that provide synthetically useful products in high yield with excellent ee. In the next budget year, we will continue to pursue this chemistry.