Reports: DNI752705-DNI7: Novel Polymeric Architectures from Monomers with Multiple Reactive Centers
Qianli Rick Chu, PhD, University of North Dakota
The synthesis of new materials is an essential element in advancing modern science and technology. Emerging two-dimensional (2D) polymers are a valuable synthetic target for predicted properties of thermal/chemical stability and mechanical strength because the polymeric structure do not automatically fall apart even if several covalent bonds are broken. Meanwhile, 2D polymers are theorized to be stronger than the traditional polymers since each monomer is connected with its neighbors by multiple covalent bonds in an organized way. Such strong and lightweight materials (SLIM) have potential application in making the next generation of fuel-efficient transportation on all scales, from aircrafts to automobiles.
2D Polymer is constructed with monomers containing more than two reactive centers. The challenge lies in synthesizing a 2D polymer by the classical one-pot approach because it can potentially cross-link between the monomers. Recently, our research team has successfully synthesized 2D polymers by using a ‘pre-organization’ method observed in nature to build biological polymers such as DNA. The initial step requires the supramolecular self-assembly of a reversible non-covalent complex with a well-defined structure. In the subsequent step, formation of covalent bonds irreversibly locks the assembly, resulting in 2D macromolecules.
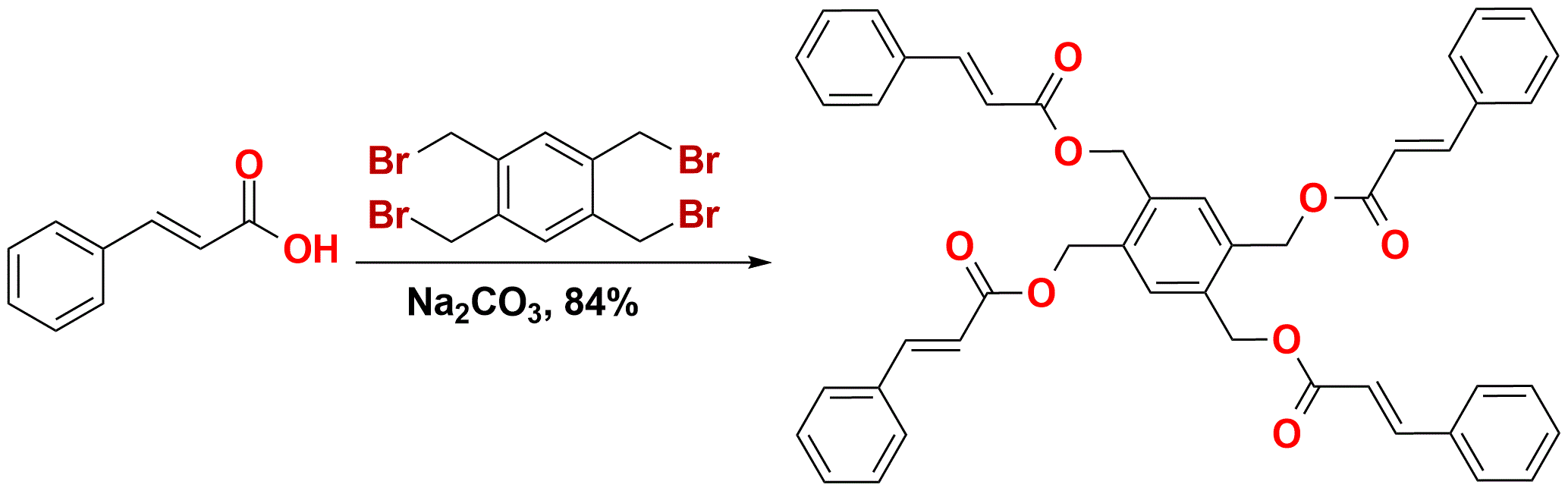
Scheme 1. Synthesis of the monomers I from cinnamic acid and 1,2,4,5-tetrakis(bromomethyl)benzene.
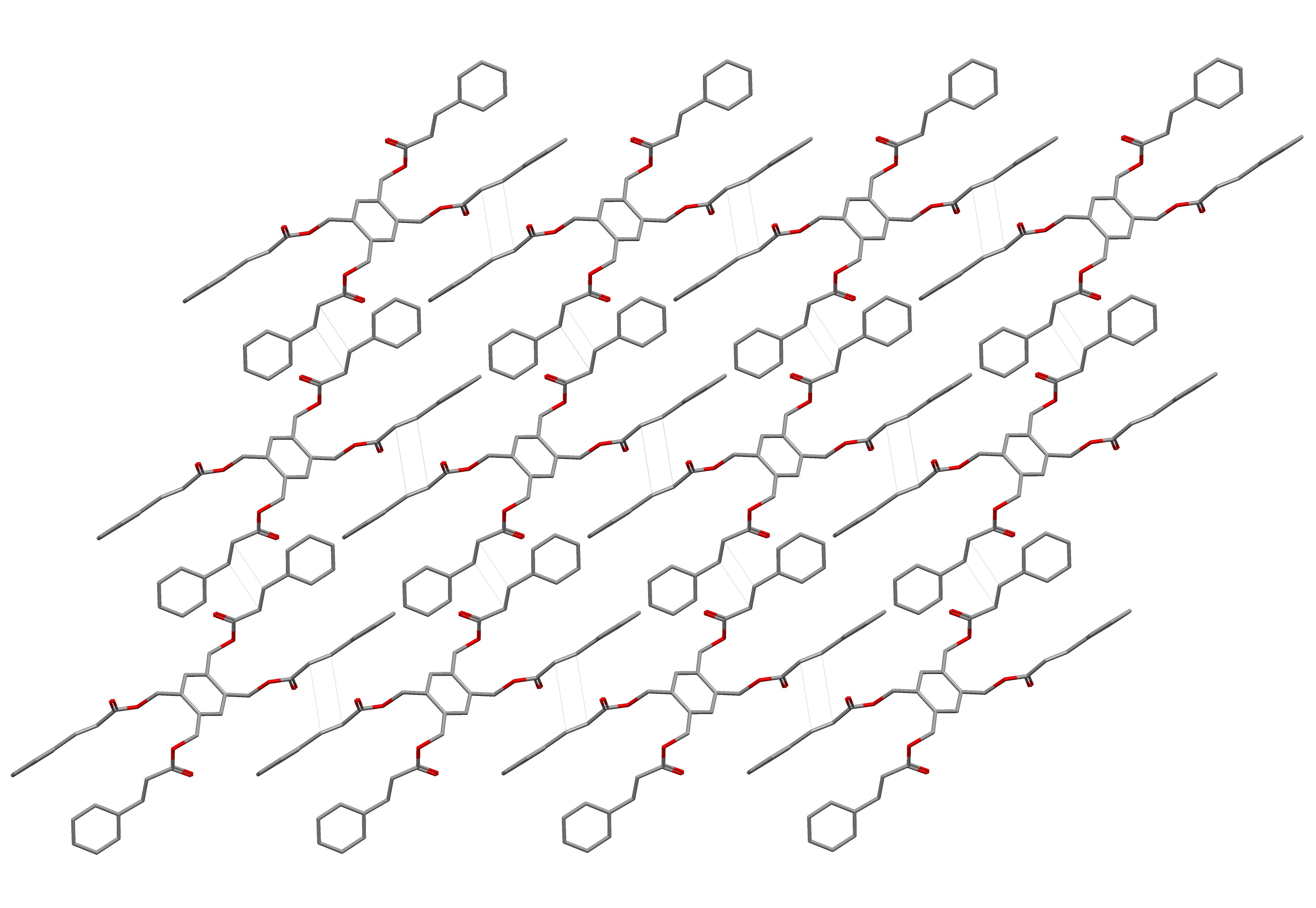
The above monomer I was synthesized by linking four cinnamic acid molecules together with 1,2,4,5-tetrakis(bromo-methyl)benzene. The monomer powder I polymerized under sunlight within 24 hours in quantitative yield. To understand the solid-state polymerization, single crystals of the four-armed symmetric monomer I were obtained. Without further processing, the powder XRD pattern of the synthesized monomer I was nearly identical to that of the ground single crystals; ultimately, this proved the powder to be micro-crystals and not an amorphous solid. The powder XRD results showed that the crystal structure of the monomer I was suitable to analyze and interpret the solid state polymerization of both the crystals and powder. The crystal structure completed a two-dimensional assembly based on complementary π-π interactions of the conjugated arms between the nearest monomers (Figure 1). The four reactive aliphatic C=C bonds in each monomer were all parallel to the double bonds in the closest four adjacent monomers, respectively. The distances between the reactive sp2 hybridized carbons in the neighboring monomers were approximately 3.9 Å. In the presence of light, the [2+2] cycloaddition locked the two-dimensional assembly into a covalently bonded two-dimensional polymer.
Figure 1. Crystal structures of the monomers I: the 2D assembly of the monomers showing the intermolecular [2+2] polymerization is favorable (the dotted lines showing where the new C-C bonds form).
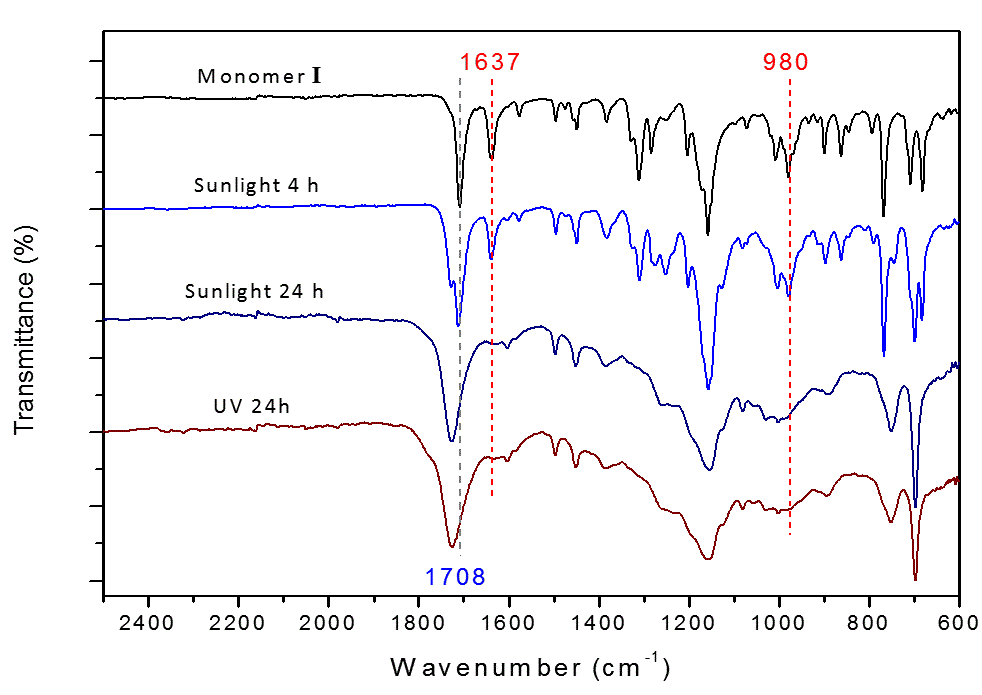
Although 2D polymers are interesting synthetic targets and have great potential for applications, the discovery and development of the 2D polymer have lagged behind other classic polymers, such as linear and cross-linked polymers. One persistent challenge is the characterization of 2D polymers due to poor solubility. In this study, the polymerization process was monitored with respect to time using FT-IR. The IR spectra in Figure 2 show that the characteristic absorption peak of C=O stretching at 1708 cm-1 shifted to 1726 cm-1 in 24 hours due to the de-conjugation of the carbonyl group. The disappearance of C=C stretching (1637 cm-1) after the reaction was consistent with completion of the [2+2] photopolymerization. The polymer was also successfully obtained through UV irradiation. The IR spectra of polymers obtained from the two different light sources were nearly identical.
Figure 2. FT-IR spectra showing the [2+2] photopolymerization completed by using sunlight or UV irradiation
A comparison of the solid state 13C NMR spectra of the monomer I and polymer IP confirmed the formation of the 2D polymer by showing the disappearance of two olefin peaks at 118 and 146 ppm concurrent with the appearance of peaks from cyclobutane around 44 ppm. Given the ester structure of the 2D polymer, hydrolysis offers another facile way to confirm the macromolecular structure. The α-truxillic acid (head-to-tail dimer of cinnamic acid) was isolated as a hydrolysis product in 72% yield after column chromatography that directly revealed the newly formed C-C bonds. The results of hydrolysis unequivocally confirmed the polymerization and displayed excellent stereoregularity of the [2+2] photopolymerization because only one of the five possible stereoisomers of truxillic acid was observed.
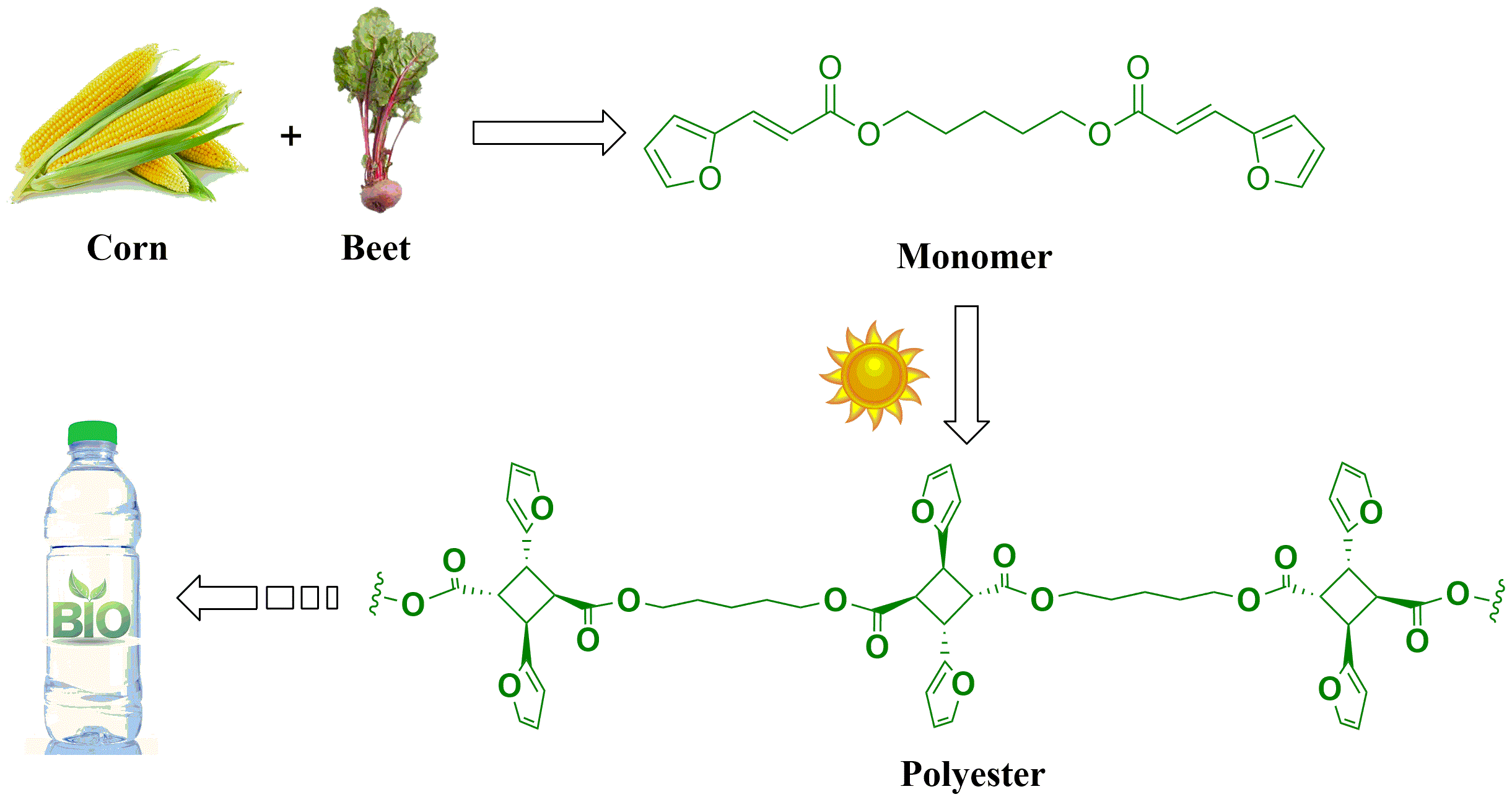
Our seminal 2D polymer paper has been published in Macromolecules in 2015. We are currently working with our collaborators to further study the properties of the novel polyester for future applications. Another recent exciting development in my research group is that we were able to synthesize a novel linear polyester from furfural and its derivatives (Figure 3). In this case, the monomer with two reactive C=C double bonds was synthesized and used to construct the linear polymer by a solvent-free photoreaction in sunlight. Similar to polyethylene terephthalate (PET), which is widely used in beverage bottles, the novel polyester contains alternating rigid and flexible moieties in the polyester chain. The corresponding manuscript has been published in Green Chem. and it will be featured as a cover story of the October issue in 2015.
Figure 3. Linear Polyester Synthesized from Furfural-based Monomer by Photoreaction in Sunlight
This ASC PRF Doctoral New Investigator (DNI) grant has helped the PI’s research team in the first steps towards their long-term research goal - to make advances that will lead to the next generation of functional polymeric materials by using monomers with multiple reactive centers. The novel linear, ladder, and 2D polymers achieved with the support of this grant are of great interest for their basic scientific richness and potential applications. This funding helped three graduate students and six undergraduates in gaining hands-on research experiences and publishing articles together in this promising research area during the past 32 months. The PI received his tenure and was promoted to Associate Professor, and one of his graduate students won the Roland G. Severson Graduate Research Scholarship this year.