Reports: DNI352325-DNI3: Cation-Crown Ether Interactions to Control Ligand Hemilability and Catalyst Function
Alexander J. M. Miller, University of North Carolina (Chapel Hill)
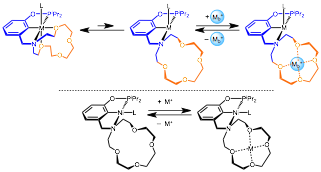
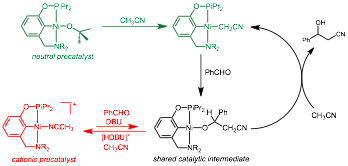
Alexander J. M. Miller, University of North Carolina (Chapel Hill)


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society