Reports: UNI153983-UNI1: Synthesis and Cycloadditions of Alkyl-substituted Siloles and Exploration of Strained Bicyclic Silane Reactivity
Thomas Charles Coombs, PhD, University of North Carolina at Wilmington
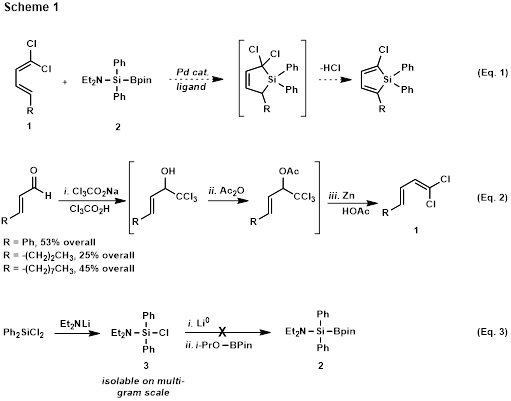
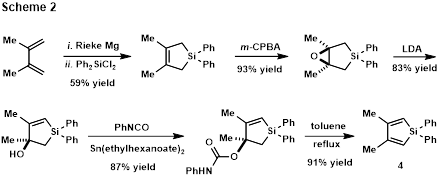
Until the Scheme 1 chemistry has been worked out, alternate approaches to siloles are being utilized. 3,4-Dimethyl-substituted silole 4 has been synthesized following Pagenkopf’s protocol[3] as shown in Scheme 2. This 5-step synthesis is scalable and 1.15 grams of 4have been synthesized.
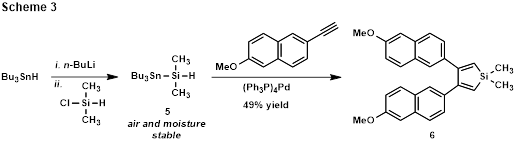
A more direct approach to 3,4-disubstituted siloles has also been explored using Ikenaga’s palladium-catalyzed [2+2+1] cycloaddition of terminal alkynes and (tributylstannyl)dimethylsilane (5), a dimethylsilylene precursor (Scheme 3).[4] In contrast to the dialkylamino-substituted silylboronic esters, the (tributylstannyl)dimethylsilane is moisture stable and easily synthesized. Silole 6 has been synthesized from 2-ethynyl-6-methoxynaphthalene in 49% yield via this protocol, and the synthesis of alkyl-substituted siloles will soon be examined.
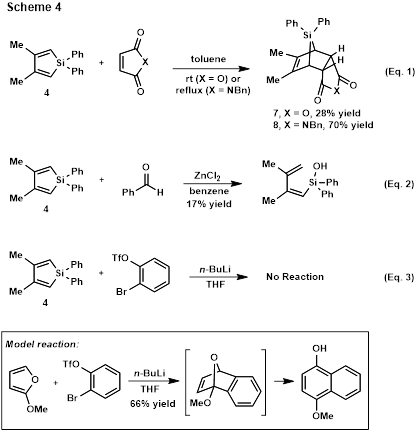
Diels-Alder cycloadditions of silole 4 have provided access to known tricyclic silane 7[3] and novel N-benzylmaleimide-derived silane 8 for further use in reactivity studies (Scheme 4, Eq. 1). However, attempts to carry out hetero-Diels-Alder reactions have only resulted in hydrolytic ring-opening of the silole (Scheme 4, Eq. 2). Attempted Diels-Alder reactions with benzyne have also failed (Scheme 4, Eq. 3), despite successful control reactions (Scheme 4, inset).
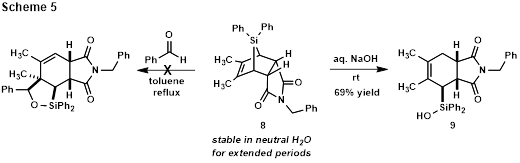
To investigate the reactivity of strained tricyclic silane 8, a series of conditions was explored. The first examined the possibility of a strain-release reaction with benzaldehyde, but even after refluxing in toluene for extended periods, silane 8 proved stable and unreactive (Scheme 5). Following precedent from Woerpel’s studies[5] of strained silacyclopropanes, a parallel screen for reactivity with benzaldehyde using NaOMe, CuI, CuBr2, and tetrabutylammonium difluorotriphenylsilicate (TBAT) as catalyst or initiator was conducted. However, no reaction, decomposition, or hydrolytic C-Si bond cleavage to produce silanol 9 was observed in all cases, even in the presence of molecular sieves. Stability studies showed that silane 8 is stable for hours in water, but reacts quickly with aqueous NaOH to produce silanol 9.
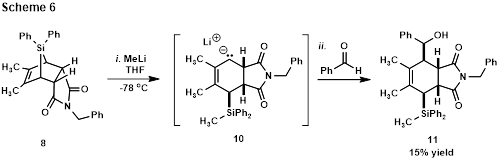
Gratifyingly, addition of MeLi to compound 8 at -78 oC resulted in ring-opening C-Si bond cleavage to generate a new carbanion that was intercepted in a C-C bond-forming reaction with benzaldehyde to produce alcohol 11. The relative stereochemistry of 11 is not yet assigned, but reaction from the convex face of the bicycle is likely. The low yield is attributed to the instability of the intermediate anion (10), as TLC shows that ring opening occurs immediately upon MeLi addition.
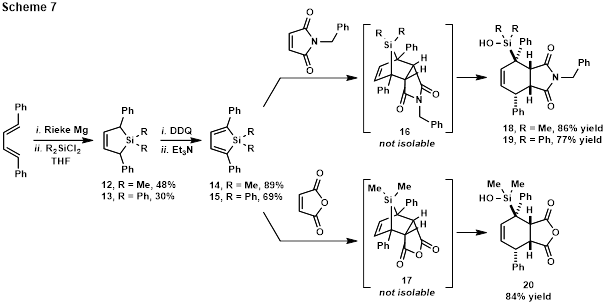
The possibility of stabilizing the anion by incorporation of an aromatic substituent was next pursued. Synthesis of the requisite dimethylsilyl-substituted silole (14), a known compound,[6] and the novel diphenylsilyl-substituted analog (15) is shown in Scheme 7.
Diels-Alder reaction of dimethylsilyl-substituted silole 14 with N-benzylmaleimide or maleic anhydride afforded ring-opened compounds 18 and 20 following column chromatography. Testing the hypothesis that replacing the methyl groups on silicon with bulkier phenyl groups might inhibit hydrolysis, silole 15 was reacted with N-benzylmaleimide. Following chromatography, only ring-opened hydrolysis product 19 was isolated. Notably, however, TLC indicates the presence of a product significantly less polar than 19 (presumably 16) prior to chromatography. This compound is stable in solution for extended periods.
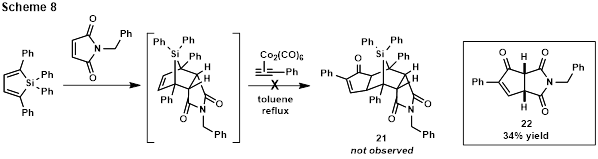
Characterization and the possibility of intercepting the initially-formed Diels-Alder adduct in situ are currently being explored. Interestingly, though an attempted Diels-Alder/intermolecular Pauson-Khand reaction sequence failed to deliver silane-fused cyclopentenone 21 (Scheme 8), N-benzylmaleimide-fused cyclopentenone 22(inset) was observed. Intermolecular cobalt-mediated Pauson-Khand reactions are limited in scope, and maleimides do not appear to have been previously reported as olefin reactants. This unexpected result is currently being studied to determine its full potential.
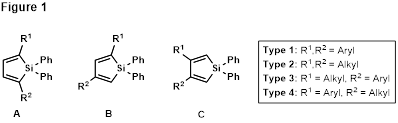
Given the striking difference in susceptibility to ring-opening hydrolysis observed between bridged tricyclic silanes 8 and 16/17, a structure-activity analysis is warranted to explore the relationship between substitution pattern/nature and stability/reactivity. Siloles to be synthesized and reacted with both maleic anhydride and N-benzylmaleimide are shown in Figure 1. Employing the silanol hydrolysis products in Hiyama-Denmark coupling reactions or further allyl silane chemistry could lead to the stereocontrolled production of densely-substituted polycyclic ring systems.
This research project has faced many challenges during the initial phase of investigation, but much has been learned about the stabilities of important reagents needed for the synthesis of variously-substituted siloles, and of several polycyclic silanes whose reactivity is to be explored and developed. To date, four undergraduate students and one graduate student have been involved with the project. Two of the undergraduate students have now joined the UNCW Master’s program and a third was accepted into a PhD program. This project has provided excellent training opportunities for students interested in exploring synthetic organic chemistry as a career option, and has also provided the PI with experience in mentoring new students and directing his first research program.
References:
[1] Z. Wang, S. Campagna, G. Xu, M. E. Pierce, J. M. Fortunak, P. N. Confalone, Tetrahedron Lett. 2000, 41, 4007-4009.
[2] T. Ohmura, K. Masuda, H. Furukawa, M. Suginome, Organometallics 2007, 26, 1291-1294.
[3] A. C. Stevens, B. L. Pagenkopf, Org. Lett. 2010, 12, 3658-3661.
[4] K. Ikenaga, K. Hiramatsu, N. Nasaka, S. Matsumoto, J. Org. Chem. 1993, 58, 5045-5047.
[5] A. K. Franz, K. A. Woerpel, Acc. Chem. Res. 2000, 33, 813-820.
[6] T. Ohmura, K. Masuda, I. Takase, M. Suginome, J. Am. Chem. Soc. 2009, 131, 16624-16625.