Reports: UR1054780-UR10: Understanding and Utilizing Block Copolymer Templates for the Preparation of Bimetallic Catalysts for Fuel Cell Applications
David Rider, PhD, BS, Western Washington University
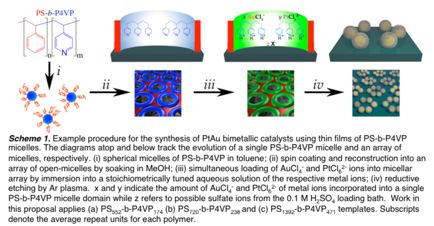
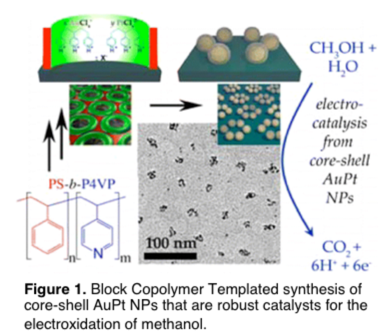
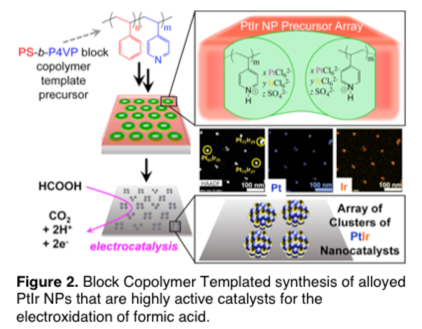
David Rider, PhD, BS, Western Washington University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society