Reports: DNI354504-DNI3: Electron-Donating Carboranyl Ligands for Iron-Catalyzed Hydrocarbon Oxidation
Dmitry V. Peryshkov, PhD, University of South Carolina
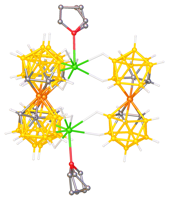
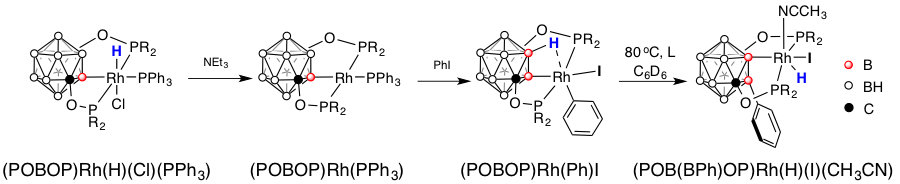
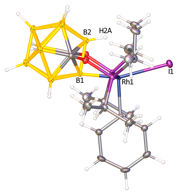
The close contact between the metal center and the vicinal
boron atom and the uniquely high strain of cage-metal bond in (POBOP)Rh(Ph)(I) led us to the
hypothesis that Rh can easily activate the adjacent B PRF grant is critical in supporting the PI`s career development
by funding our work on the use of icosahedral borane clusters as electron-donating
ligands for transition metals. This project grew into the initially
unanticipated area of chelating pincer complexes. These results will serve as
the foundation for the PI`s research program that aims to develop novel
catalysts featuring ligand-metal cooperativity. As a result of Year 1, the
manuscript, co-authored with the graduate students, describing the uncovered
reactivity in the POBOP-Rh system is under review. We are expanding the scope
of this project by the synthesis of analogous pincer complexes of the first row
transition metals, including iron. The graduate students supported by PRF fund obtained
extensive training in synthetic organometallic chemistry, work with
air-sensitive compounds, and related safety practices.