Reports: DNI554706-DNI5: Role of Surfaces and Surface Charge on Hydrate Nucleation and Adhesion
Vaibhav Bahadur, PhD, The University of Texas At Austin
Summary:
Over the past year, grant funds have been used to build four experimental setups and conduct experimental studies. Two PhD students and one undergraduate student have been supported. This work has resulted in 1 journal article (published) and 2 articles accepted in international conferences.
Introduction:
Formation of natural gas hydrates in deepwater infrastructure is a big challenge faced by the petroleum industry. Hydrates have other applications in carbon sequestration, natural gas transport and desalination. This work aims to uncover the role of surface chemistry and surface charge on hydrate nucleation and adhesion. Year 1 progress can be described in terms of 3 studies.
Study 1 (completed): Fundamental studies on surface electric charge-controlled nucleation
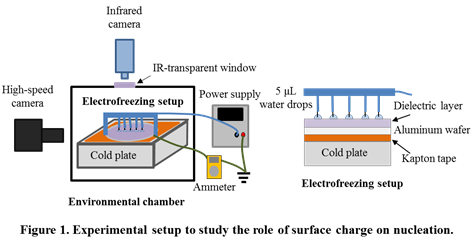
Electrofreezing is the electrically-induced nucleation in a supercooled liquid. As a precursor to studying the role of surface charge on hydrate nucleation, an experimental setup (Figure 1) was built to study the influence of surface charge on ice nucleation from supercooled water droplets. The objective was to gain insights into statistical measurements of nucleation and also train a graduate student for future, more complex experiments with hydrates.
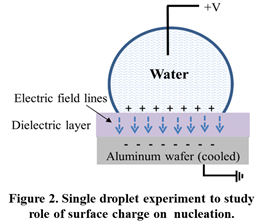
In this study, there is no electric field inside the liquid, which rests on a dielectric layer (Figure 2). Instead, there is an interfacial electric field and charge buildup at the solid-liquid interface. This situation contrasts previous electrofreezing studies, which used bare electrodes, involved current flow, and had a volumetric electric field inside the liquid. Infrared and high-speed visualizations were used to quantify electrofreezing. Ultra-high electric fields of up to 80 V/µm were applied, which are one order of magnitude higher than in previous studies.
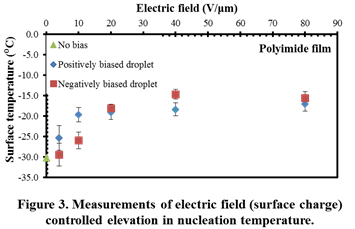
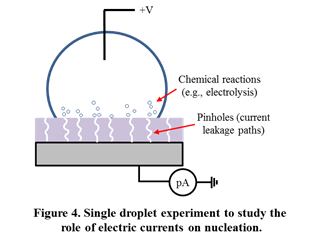
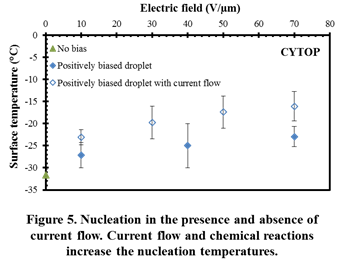
The results facilitate an in-depth understanding of various mechanisms influencing nucleation of water and hydrates. Firstly, it was seen that surface charges and interfacial electric fields alone can significantly elevate freezing temperatures by more than 15 °C, in the absence of current flow (Figure 3). Secondly, the magnitude of electrofreezing-induced temperature elevation was seen to saturate at high electric field strengths. Thirdly, the polarity of the interfacial charge did not significantly influence electrofreezing. Overall, it was seen that electrofreezing-based nucleation predominantly occurs at the three-phase boundary and not in other parts of the solid-liquid interface. Through careful electrofreezing measurements on dielectric layers with pinholes (Figure 4), which allow current flow, the individual role of electric fields and electric currents on electrofreezing was isolated. Much higher electrofreezing temperatures were observed in the presence of electric currents (Figure 5). Overall it is concluded that surface charges as well as electric currents influence nucleation; however the physical mechanisms are very different.
Study 2 (completed): Fundamental studies on role of surface chemistry on nucleation
As a first step to studying the role of surface chemistry on hydrate nucleation and adhesion, experiments were conducted to study surface chemistry controlled-ice nucleation. The role of water salinity in conjunction with surface chemistry was also studied. It is important to note that hydrate nucleation kinetics show a strong dependence on salinity. An understanding of saltwater ice nucleation is also important, since marine ice poses challenges to the petroleum industry in norther latitudes.
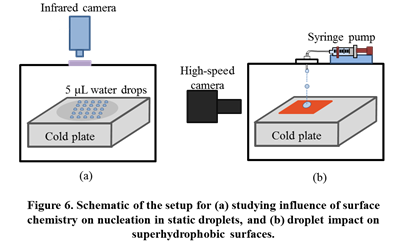
Experiments were conducted to understand the influence of surface chemistry on heterogeneous saltwater ice nucleation and propagation under static (stagnant water) and dynamic (liquid impact) conditions (Figure 6). These experiments provide insights into ice/hydrate adhesion, by measuring the utility of superhydrophobic surfaces for saltwater icephobicity. All these experiments were conducted with pure water and two sodium chloride solutions, which represent the salinity of seawater and briny produced water.
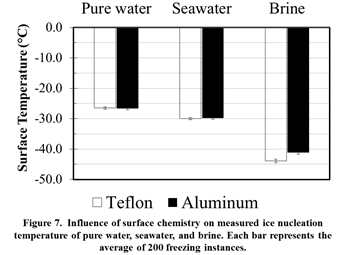
The static droplet experiments provide insights into the mechanisms involved in heterogeneous freezing of saltwater. Firstly, it was verified via high-speed imaging that nucleation always originates at the three-phase contact line for all fluids. Interestingly, surface chemistry was not seen to have a measureable influence on the heterogeneous freezing temperatures of pure water and saltwater (Figure 7). However, the presence of salt slowed down the nucleation front propagation velocity significantly.
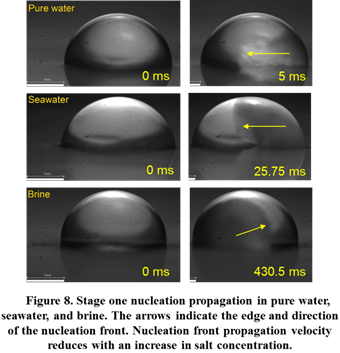
Saltwater droplet impact dynamics on a superhydrophobic surface was also different from pure water. Saltwater droplets retracted more and a greater fraction of impacting liquid was repelled from the surface. Modeling showed that differences in thermophysical properties of liquids is not the primary reason for the difference in the impact dynamics. It was proposed that the greater bounciness of saltwater droplets is due to slower ice nucleation propagation kinetics. This hypothesis was verified by high speed imaging-based measurements of propagation velocities of the initial freezing stage in pure water and saltwater droplets (Figure 8). Overall, these experiments indicate that superhydrophobic surfaces will offer better resistance to impact icing with saltwater than pure water and can remain useful at temperatures as low as -40 °C.
Study 3 (ongoing): Studies on the role of surface chemistry and surface charge on tetrahydrofuran and methane hydrate nucleation
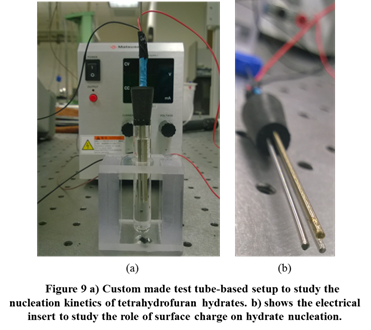
Two additional experimental setups are being developed to characterize nucleation in hydrate systems. Figure 9 shows a setup to study the role of surface chemistry and electric charge on nucleation of tetrahydrofuran (THF) hydrates. THF hydrates form above 0 °C at atmospheric pressures; the simplicity associated with THF hydrate formation allows detailed, upclose measurements of nucleation kinetics. This test setup enables 20 simultaneous experiments on THF hydrate formation in test tubes, and will provide statistically relevant results.
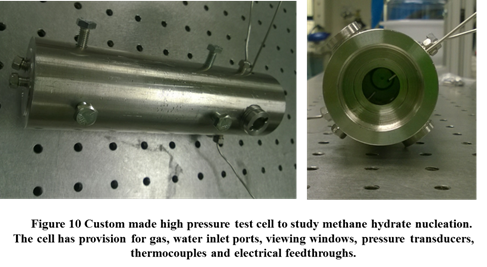
Figure 10 shows a custom-built experimental facility which is being developed to study the role of surface chemistry and surface charge on methane hydrate formation. This setup consists of a high pressure cell (~ 100 bar) which will contain natural gas and water; the entire cell is immersed in a cold bath (-10 ° C). The influence of surface chemistry will be quantified by coating the insides or via the use of chemically functionalized metal foams inside the cell. The role of surface charge will be isolated by applying an interfacial electric field using electrical feedthroughs. These experiments will be followed by measurements of the adhesion energy of hydrates to a surface.