Reports: DNI1054697-DNI10: Understanding the Interfaces Between Iron and Iron-Carbides
Christopher R. Weinberger, PhD, Drexel University
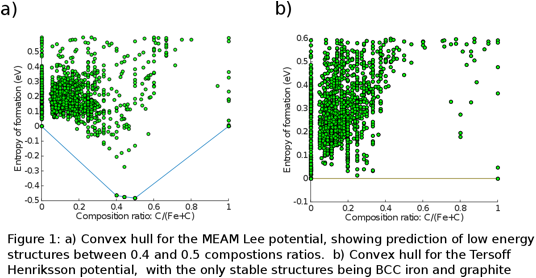
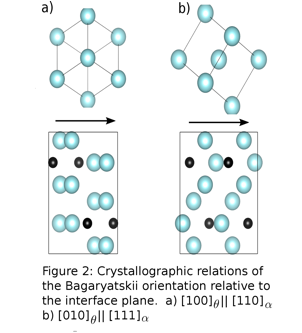
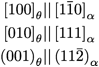
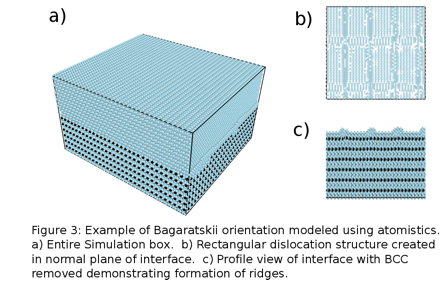
Christopher R. Weinberger, PhD, Drexel University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society