Reports: ND1053140-ND10: Nanofabrication of Porous Membranes for Separations of Gaseous Energy Carriers Under Conditions of Single-File Diffusion
Kirk J. Ziegler, University of Florida
Sergey Vasenkov, University of Florida
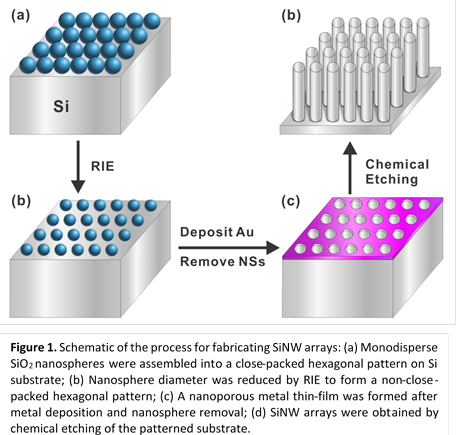
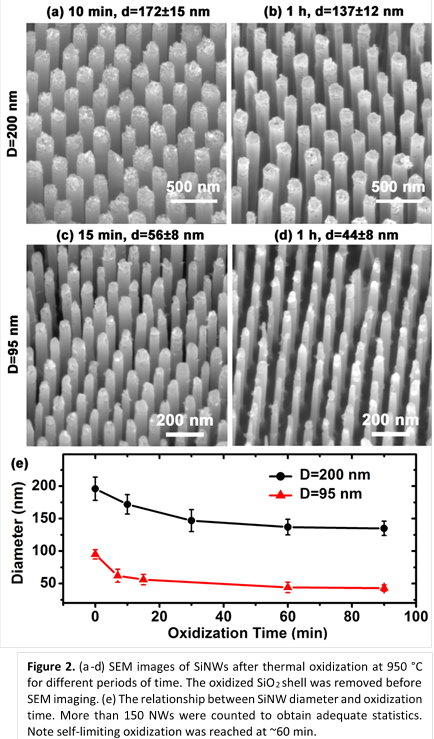
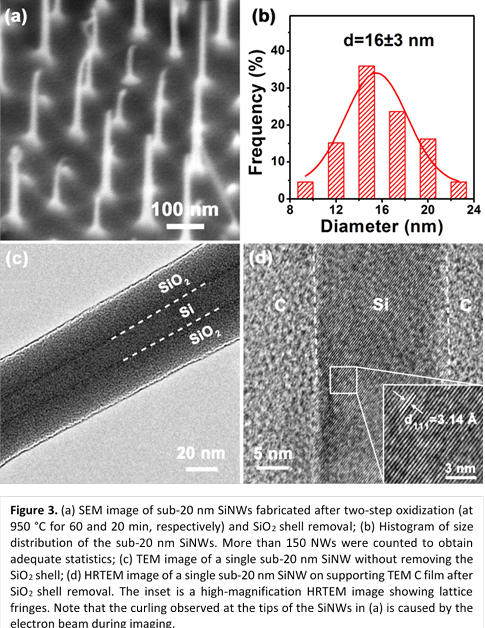
A two-step oxidization technique was used to fabricate
sub-20 nm SiNW arrays, where a second oxidization was performed following the removal
of the SiO2 shell. SiNWs with an initial diameter of 95 nm were
used. The results after the second oxidization are shown in Figure 3. The
NWs still have uniform spacing and good vertical alignment. The average
diameter of the NWs was 16 nm, as shown in the histogram. NWs with average
diameters smaller than 16 nm can be obtained by using a longer duration for the
2nd oxidization (>20 min).
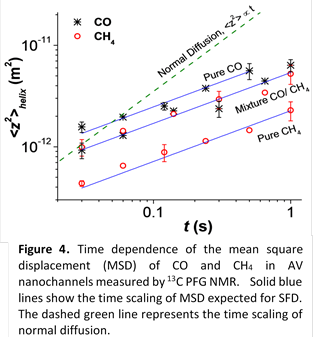
First observation of single-file diffusion for molecular mixtures. In
membrane-based separations of gas mixtures, more than one gas component is
expected to be present in membrane channels. Hence, studies of the feasibility
of gas separations under SFD conditions requires an ability to observe and
investigate single-file diffusion of molecular mixtures. During the last year
of the project, C-13 PFG NMR at a high magnetic field of 17.6 T and large
magnetic field gradients up to 30 T/m was applied to study diffusion of C-13
labeled CO and CH4 in L-Alanyl-L-Valine (AV) dipeptide nanotubes.
Both mixed and pure gases were used in diffusion studies. AV nanotubes were
selected as a model nanotube system capable of inducing SFD conditions for
small gas molecules.
Ms. Akshita Dutta, a University of Florida PhD student working on the project,
has developed an expertise in the area of diffusion studies of nanoporous
materials as well as in the application of high field NMR in these studies.