Reports: UR353707-UR3: Synthesis and Reactivity of Molybdenum and Tungsten Carbon Dioxide Complexes
Peter M. Graham, PhD, Saint Joseph's University
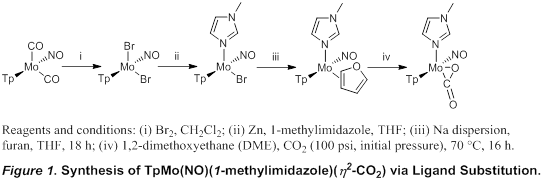
Since CO2 coordination can be a prerequisite for CO2 activation, the preparation and study of isolable CO2 complexes is of great interest. Side-on (η2) coordination is often observed for monomeric complexes of CO2 which are most frequently prepared by substitution of a labile ligand. Our work in this area began with the report that η2-CO2 complexes of molybdenum and tungsten can be prepared via thermal displacement of a substitutionally labile aromatic ligand under slightly elevated pressures of CO2. Figure 1 depicts the synthesis of TpMo(NO)(1-methylimidazole)(η2-CO2) (Tp = tris(pyrazolyl)borate) by displacement of furan from TpMo(NO)(1-methylimidazole)(η2-furan).
As we endeavored to prepare η2-CO2
complexes of this type featuring σ-donors (Ld) beyond 1-methylimidazole,
several features of this methodology hindered our progress. First the
preparation of the η2-furan complex from TpMo(NO)(1-methylimidazole)Br
requires reduction with sodium dispersion and typically results in low yields (~25%).
More importantly, when the complex TpMo(NO)(Ld)Br features a
σ-donor less donating than 1-methylimidazole, the complex can become too
electron deficient for an η2-furan complex to be isolable. Consequently,
a new synthetic route to η2-CO2 complexes was pursued.
Dihapto carbon-dioxide complexes can sometimes be prepared
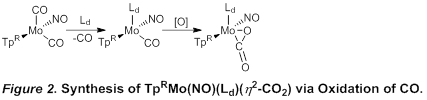
by the oxidation of a carbonyl ligand. Thus we pursued a simplified synthetic
strategy (Figure 2). A carbonyl ligand can be thermally liberated from TpRMo(NO)(CO)2
(TpR = Tp or Tp*, tris(3,5-dimethylpyrazolyl)borate) in the
presence of a σ-donor ligand to give TpRMo(NO)(Ld)(CO).
Using this method, fifteen complexes featuring Ld = P(OMe)3,
PPh3, P(p-tolyl)3, PMe3,
3-fluoropyridine (F-Py), 3-chloropyridine (Cl-py), pyridine (Py),
4-dimethylaminopyridine (DMAP), 1-methylimidazole (1-MeIm), 1,3-dimethylimidazol-2-ylidene
(NHC), have been prepared and purified by column chromatography. Several of
these complexes are air sensitive. In some cases, IR spectroscopy indicates
that small quantities of η2-CO2 complex are formed
during heating if oxygen is not rigorously excluded. Consequently, the best
results were obtained by performing thermolysis in a sealed pressure tube. The
analogous tungsten complexes cannot be prepared via this method due to the
reticence of TpRW(NO)(CO)2 to release CO upon heating. However,
we are currently pursuing photochemical methods to address this limitation.
The range TpRMo(NO)(Ld)(CO) is
constrained by the steric and electronic properties of Ld. For Tp,
complexes featuring alkyl phosphines larger than PMe3 could not be
isolated, including PEt3 and P(n-Bu)3. For Tp* the
more sterically demanding ligand platform means that a handful of complexes
isolable for Tp were not isolated, including PPh3, NHC, and Cl-Py. On
the other hand, the added electron-donating ability of the Tp* chelate allowed
a 3-fluoropyridine (F-Py) complex to be isolated for Tp*, while the analogous Tp
version could not be isolated.
Oxidation of these carbonyl complexes to give TpRMo(NO)(Ld)(η2-CO2)
is accomplished using a range of mild oxidants including cumene hydroperoxide, tBuOOH,
or oxygen gas. Most commonly, a hydroperoxide gives the best yields of η2-CO2
complex, usually giving isolated yields between 40 and 80%. In the case of Ld
= DMAP, the hydroperoxide oxidants produces low yields of η2-CO2
complex. However, oxygen proves to be the best oxidant giving Tp*Mo(NO)(DMAP)(η2-CO2)
in 44% and TpMo(NO)(DMAP)(η2-CO2) in 38% isolated
yield after chromatography.
Dihapto-CO2 complexes are isolable for a range
of complexes featuring σ-donors that are phosphines, pyridines, imidazoles,
and N-heterocyclic carbenes. Carbon dioxide complexes could not be
isolated for the more electron-deficient Ld (PPh3, P(OMe)3,
P(p-tolyl)3). For these complexes, cumene hydroperoxide did
not oxidize the requisite complex. Stronger oxidants such as PhIO did react,
but CO2 complexes could not be isolated or definitively identified in
situ via spectroscopic methods. Since oxidation is a proven decarbonylation method
via formation of CO2, it seems likely that these complexes are
simply too electron deficient to maintain coordination of CO2. Heretofore,
the least electron rich η2-CO2 complex isolated is
TpMo(NO)(Cl-Py)(η2-CO2) based on IR
spectroscopy and oxidation potential as measured by cyclic voltammetry.
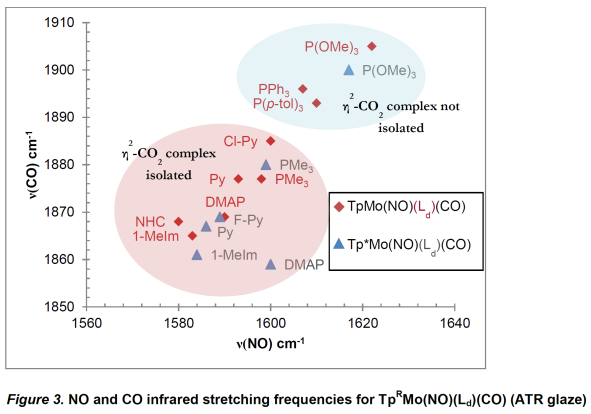
Unsurprisingly, carbonyl and nitrosyl stretching
frequencies are excellent indicators of the electron density of TpRMo(NO)(Ld)(CO)
complexes. Complexes featuring νCO > 1893 cm-1
and νNO > 1607 cm-1 did not result in isolated
η2-CO2 complexes upon oxidation. However, all
isolated TpRMo(NO)(Ld)(CO) complexes featuring νCO
and νNO lower than these have led to isolable η2-CO2
complexes upon oxidation (Figure 3).
Dihapto-CO2 complexes feature nitrosyl
stretching frequencies from 1611-1633 cm-1. In contrast to the CO
complexes, the nitrosyl stretch of the η2-CO2
complex appears to be insensitive to TpR substitution, nor is an
obvious trend based on Ld clear. Certainly the electron density at
the metal is more governed by the presence of the η2-CO2
ligand than the σ-donor.
The formation of TpRMo(NO)(Ld)(CO) complexes
from η2-CO2 complexes can occur under certain
reducing conditions, usually in a few hours at room temperature. Activated
magnesium gives CO complexes at room temperature in 50-75% isolated yield after
column chromatography, but requires the addition of P(NMe2)3,
presumably as an oxygen acceptor since O=P(NMe2)3 is
detected by 31P NMR spectroscopy. The hydrides LiAlH4 and
LiBH4 also give good yields of the CO complexes. For example, TpMo(NO)(PMe3)(η2-CO2)
can be reduced to the CO complex in 75% yield by LiBH4 at room temperature.
Having synthesized eleven molybdenum η2-CO2
complexes with a range of steric and electronic features, we are currently
working to explore the reactivity of these complexes, especially the coupling
of η2-CO2 complexes with alkenes to give
acrylates.
During the first fiscal year of this grant (January
2014-August 2015), seven undergraduates have been engaged in research in my
laboratory. Two of these students were supported by the grant during the fiscal
year, one during both summers. During this period, students have presented posters
at the ACS National Meeting in Dallas (March, 2014) and Denver (March, 2015) as
well at the Mid-Atlantic Seaboard Inorganic Symposium in Philadelphia (July,
2014), and the Philadelphia Inorganic Colloquium (February 2015). Three of
these students have graduated with a B.S. in Chemistry and two of those are now
attending Ph.D. programs in Chemistry at Duke University and University of
Georgia.