Reports: DNI554217-DNI5: In Situ Characterization of Small Organic Molecule Oxidation Using Stimulated Raman Spectroscopy
Jin Suntivich, Cornell University
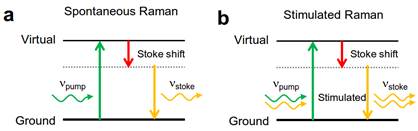
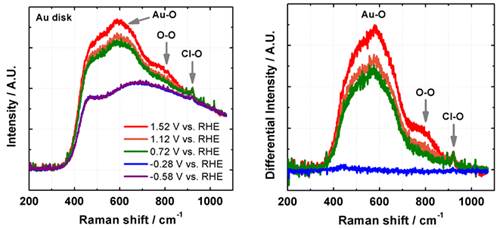
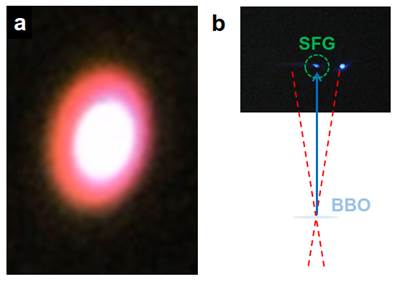
Jin Suntivich, Cornell University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society