Reports: UNI753041-UNI7: Synthesis of Ester and Amide Functionalized Polyethylene: New Polyolefin Architectures, Their Resulting Thermal and Mechanical Properties, and Controllable Depolymerization
Nicholas J. Robertson, PhD, Northland College
Year 2 Update for Synthesis of Ester and Amide Functionalized Polyethylene: New Polyolefin Architectures, Their Resulting Thermal and Mechanical Properties, and Controllable Depolymerization
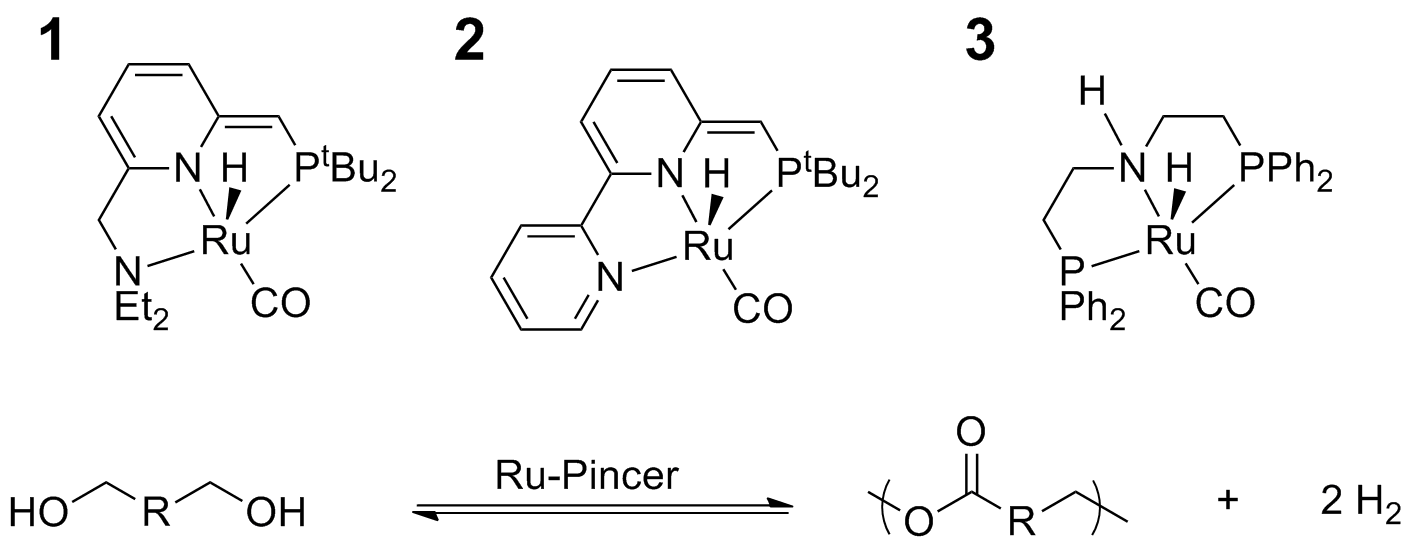
Research Summary: New ruthenium catalysts supported by pincer ligands that have been developed by Milstein and coworkers (complexes 1 and 2) and researchers at Takasago Corporation (complex 3) have demonstrated impressive capabilities for the formation of carboxylic acid derivatives via dehydrogenation or the hydrogenation of carboxylic acid derivatives. These catalysts have excellent functional group tolerance and enable transformations that commonly require stoichiometric quantities of reagents. We have found that they are effective for the polymerization diols to form polyesters and the depolymerization of polyesters to form diols (Scheme 1), representing a unique approach to preparing novel polymer architectures that can be chemically recycled. Other groups have shown that these same catalysts can couple diols and diamines to form polyamides. The extraordinary breadth of reactions that these catalysts have been used for suggests that there is great potential for them to be used in the synthesis of previously inaccessible polymer architectures. We have been focused on using inexpensive commercially available petroleum derived monomers and macromonomers to prepare new polyesters and polyamides.
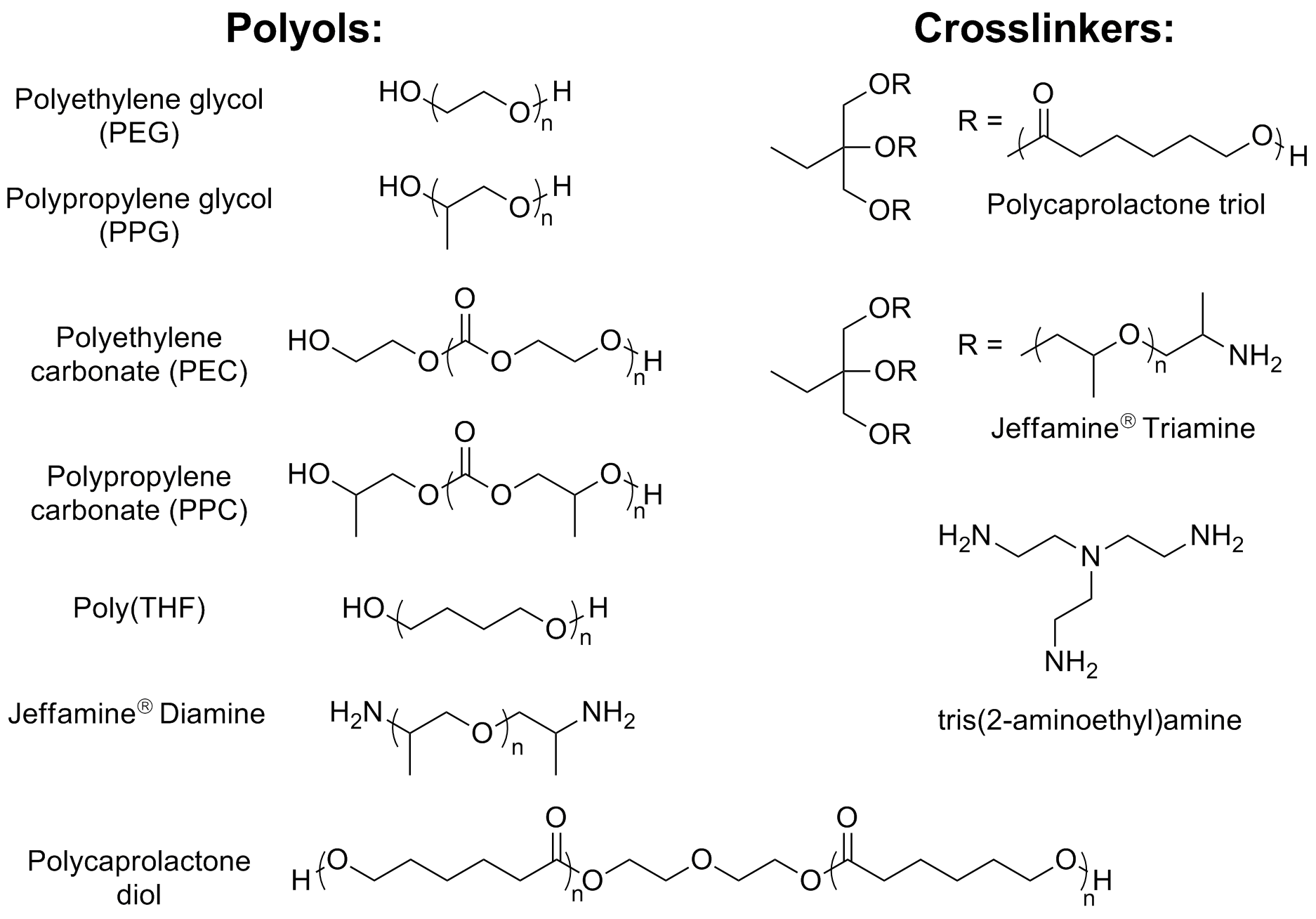
There are a wide variety of commercially available polyols that are commonly used to prepare polyurethanes through combination with isocyanates. Polyols are short length hydroxyl-terminated polymers that are commonly polyethers, polyesters or polycarbonates. While polyurethanes are remarkably useful materials with a wide variety of applications, isocyanates pose significant health risks. Therefore, it would be very advantageous to replace this component of the mixture with more benign chemicals if the mechanical properties could be maintained. A few common examples of polyols are shown in Figure 1. Different combinations of polyols lead to strikingly different mechanical properties in the resulting materials.
Notably, Huntsman Corporation has developed a class of amine-terminated polyols (Jeffamines®), which form polyureas when reacted with isocyanates. Because catalysts 1-3 can couple alcohols as well as alcohols and amines, exposing polyols or amine-terminated polyols to these catalysts could provide a new pathway to polyurethane- and polyurea-like materials, respectively, without the need for harmful isocyanates. Indeed, catalysts 1-3 exhibit reactivity toward these polyols as evidenced by hydrogen evolution and increasing viscosity, and preliminary results are promising. This reactivity also provides a facile new pathway for forming blocky polyurethanes and/or polyureas, in which the composition of the polymer backbone varies through the combination of different types of polyols (e.g., combining polyethylene glycol (PEG) with polyethylene carbonate). Furthermore, the resulting materials can all be depolymerized via hydrogenation. In addition to forming linear polymers with this methodology, using a triol or triamine, such as those shown in Figure 1, in varying ratios with the diols enables the formation of branched or crosslinked materials. These materials display significantly different properties than their linear counterparts. We are currently working to fully characterize these new materials.
While working to synthesize these materials, we have also begun to expand a pathway that we developed for the chemical recycling of post-consumer plastics such as polyethylene terephthalate (PET). We previously showed that catalysts 1 and 2 can hydrogenate PET to form benzenedimethanol and ethylene glycol. While this methodology is a useful proof-of-concept, it would be of great value to be able to derive a larger variety of chemicals from waste plastics. To achieve this we have begun studying the transesterification and transamidation via dehydrogenation with secondary alcohols and amines. Catalysts 1-3 have effectively catalyzed these types of reactions on small molecules. While transesterifications and transamidations are well known, most often they generate small molecule byproducts that must be boiled off or neutralized in order to drive the equilibrium toward completion. The dehydrogenative pathway is promising because it generates hydrogen as a byproduct, which is easily removed from a reaction mixture and can therefore aid in driving these reactions to complete conversions. Furthermore, the small molecule analogue esters are can undergo transamidation with more sterically hindered amines, opening the door to a wide variety of potential depolymerization products.
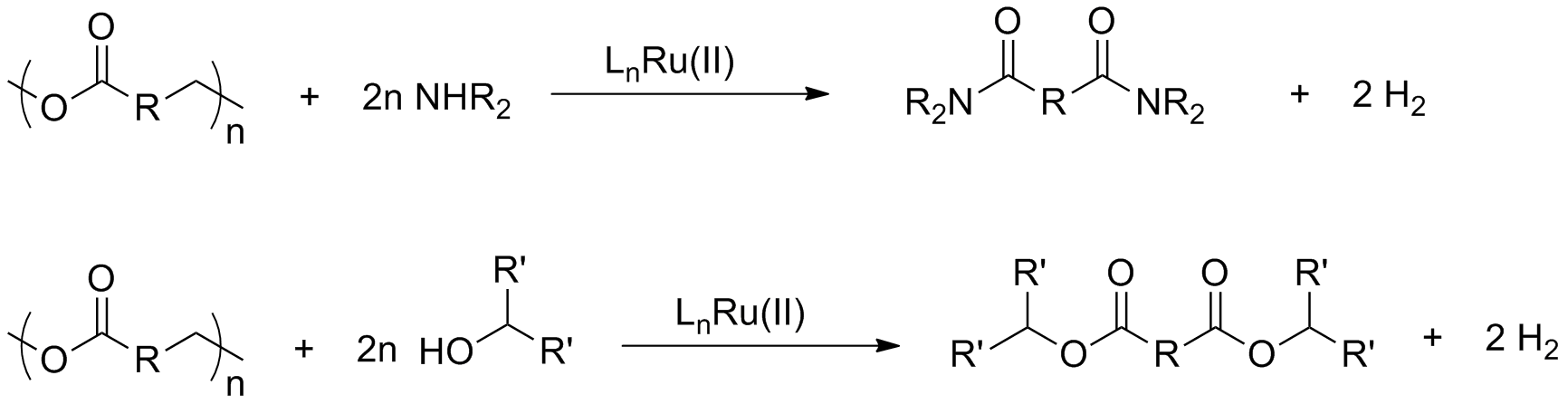
To test the feasibility of this pathway we began preliminary work on depolymerization of post-consumer PET (Scheme 2). Rapid evolution of hydrogen gas was observed and IR spectroscopy showed a significant shift of the carbonyl stretching frequency from ester to amide when reacted with amines. We are currently studying the product mixtures with NMR to further elucidate the conversion and product mixture. Upon optimizing the reaction conditions, we plan on extensively studying the substrate scope to generate as many chemicals as possible to demonstrate that petroleum-derived plastics can be used as a feedstock for the synthesis of valuable chemicals.
Broader Impacts of ACS PRF Funding: The ACS PRF Undergraduate New Investigator funding has been instrumental in the development of my personal career, but more importantly, it has enabled me to hire and work with five outstanding students to date. These students have had life-altering experiences in the lab through their research projects. All five of them have found the hands-on work and the process of research to be so enjoyable that they are now planning on pursuing graduate school. Furthermore, their work will lead to another publication in the near future and results are serving as the basis for a proposal to the National Science Foundation. Speaking from experience, this award is truly transformative for new faculty and their students at PUIs.











