Reports: DNI553684-DNI5: Separating the Effects of Location and Substituent Identity on the Acidity of Microporous Materials
David Flaherty, PhD, University of Illinois, Urbana-Champaign
Impact of Proposed Research
Zeolite catalysts provide economic and environmental benefits in chemical and petroleum industries. The production of most gasoline and a large fraction of platform chemicals (e.g., xylenes, light alkenes) utilize zeolite catalysts for hydrocarbon cracking and reforming reactions. The catalytic activity of zeolites originated from Brønsted acid sites located within the porous structures of these materials as well as on their external surfaces. These sites promote an array of reactions, analogous to those catalyzed by mineral acids such as H2SO4 and HF, yet zeolites are less hazardous and easier to contain.
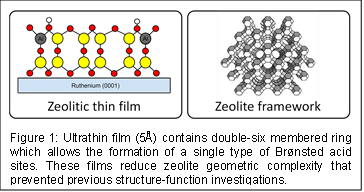
Each Brønsted acid site within a zeolite can have a distinct reactivity and selectivity, because zeolites have intricate and varied structures which can influence the strength of the acid sites (i.e., their deprotonation energies) as well as weaker interactions between reactants and walls of the porous environment (i.e., van der Waals forces). Zeolites created by wet chemistry contain a wide-variety of distinct Brønsted acid sites, which are differentiated by their location within the zeolite framework. This complexity prevents researchers from establishing simple connections between the environment of the Brønsted acid site, the deprotonation energy of the site, and its catalytic reactivity. We are working to develop quantitative relationships between these factors through investigations of zeolitic thin (5 Å) films which we grow epitaxially from metal single crystal supports (Figure 1). Our films are comprised entirely of a standard building block of commercial zeolites (a double-six membered ring) and only possess a single type of Brønsted acid site, which eliminates the complexity that prevented previous structure-function investigations.
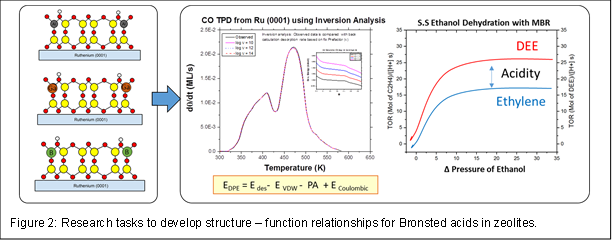
We will establish these relationships from the following tasks, which are schematically shown in Figure 2. (1) Synthesize ultrathin films with an isomorphous substituent atom (ISA), such as Al, which produces uniform acid sites. (2) Quantify each film’s acid strength by determining the deprotonation energy (DPE) using desorption kinetics obtained by temperature programmed desorption (TPD) of a series of basic molecules. (3) Perform steady state ethanol dehydration reaction using film catalysts to determine reaction rates and selectivities. (4) Conduct a similar series of experiments with films containing other ISAs (Ga, B). And (5), Establish connections between experimental DPE values and catalytic metrics.
Impact of the ACS-PRF Award on Students and the PI
This award has had a positive impact on both the students involved in the research project and the PI. Regarding students, this award has supported the research activities of one PhD student (SiWei Andy Chang) and two undergraduates (Aileen Wee, Kenneth Yun). The student worked closely with the PI to design the experimental system (described below) and to conceive the experiments. Mr. Chang presented preliminary results of this study in a poster competition for the Catalysis Club of Chicago’s (CCC) Spring Symposium in 2015, and he has also regularly attended monthly meetings of the CCC. Both graduate and undergraduate students have learned to use and analyze data from a variety of experimental methods including Auger spectroscopy, low energy electron diffraction, temperature programmed desorption spectroscopy, and molecular beam scattering.
The PI has benefitted greatly from the early support of this DNI award. This award supported an emerging research thrust in the group to relate thermodynamic properties of catalyst materials (measurable only using surface science techniques) to catalytic reactivity. The work supported here will produce several journal articles, but also methods that will be used in future studies of hydrodeoxygenation reactions and catalysts (e.g., metal phosphides). This award, therefore, has helped the PI develop ideas and tools that will form the basis of future proposals.
Preliminary Results
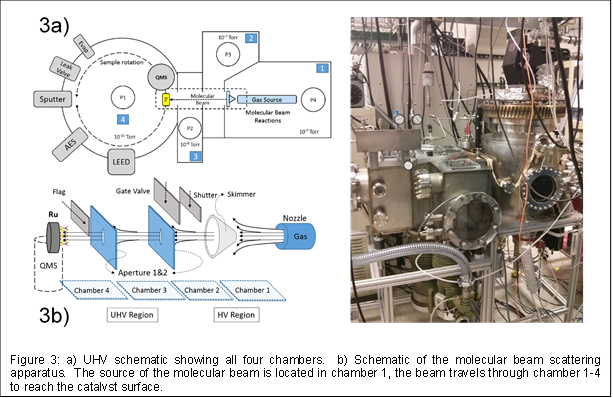
Completion of UHV Chamber: In the past year finished constructing an ultra-high vacuum (UHV) chamber with a molecular beam surface scattering apparatus. The UHV system includes an array of surface science tools (see Figure 3a) and is comprised of four individually pumped vacuum chambers. This systems allows us to investigate epitaxially grow the zeolitic films (so far, silicate and aluminosilicate), perform TPD measurements, and conduct steady-state reactions with the molecular beam.
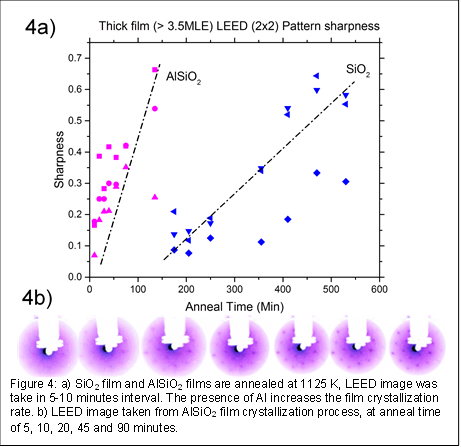
Results of Preliminary Studies: This year, we successfully developed methodology to perform TPD, established an inversion analysis program to calculate desorption energy, and synthesized SiO2 and AlSiO2 ultrathin films. Our calculated desorption energy of CO TPD was within ± 3 kJ/mol of the published data using a combination of the TPD setup and inversion analysis technique. While synthesizing the ultrathin films, we discovered that their thickness is reduced during O2 anneal at 1200 K, which can be compensated by 1) overgrowing the film during Si and Al deposition 2) decreasing the O2 anneal temperature to 1125 K, and 3) increasing the O2 pressure during the anneal process. Finally, we investigated the rate of film crystallization during the annealing process. Our preliminary results (Fig. 4) indicate that the addition of Al in SiO2 film can increase the rate of crystallization, as determined by LEED data (quantify LEED spot sharpness). Our data support the reported DFT results by other groups, suggesting that the Al-O bond (1.70 Ŗ1.73 Å) has lower bond strength than Si-O bonds (1.58 Ŗ1.64 Å). This difference creates more flexible O-Al-O and Al-O-Si bond angles to better match with Ru (0001) lattices.