Reports: DNI653476-DNI6: Spectroscopic Investigation of Vibronic Interactions in Molecules with Low Symmetry
Jinjun Liu, PhD, University of Louisville
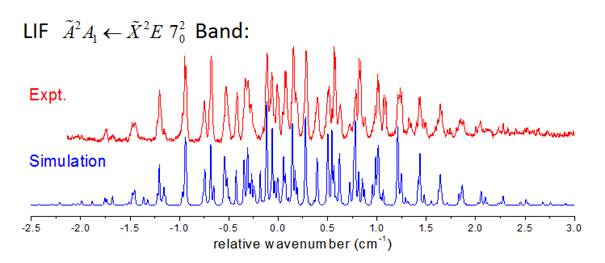
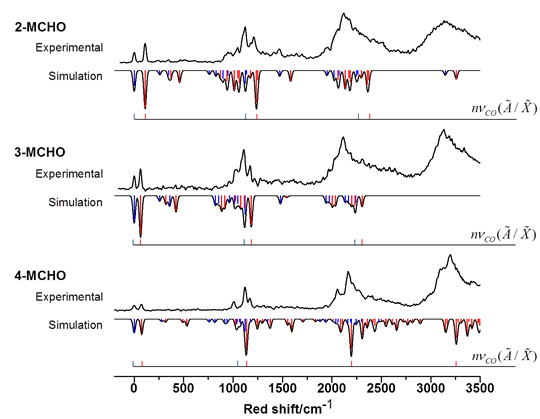
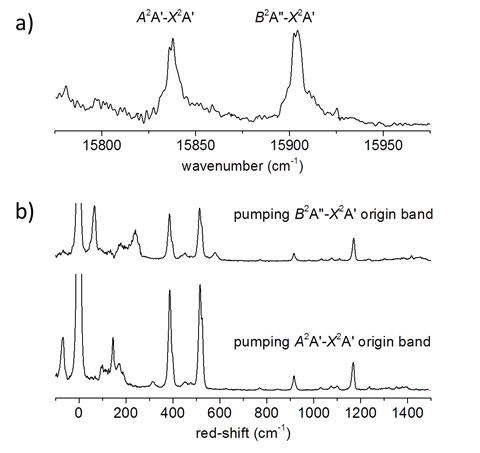
Jinjun Liu, PhD, University of Louisville


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society