Reports: UR954712-UR9: Deciphering the Coupled Diffusive and Naturally Convective Transport during Hydrocarbon Evaporation
Peter L. Kelly-Zion, PhD, Trinity University
Christopher J. Pursell, PhD, Trinity University
Overview
The goal of this project is to experimentally investigate the thermal and mass transport processes controlling the evaporation of sessile hydrocarbon drops. Through systematic experimentation during which the relative influences of the transport processes are controlled, this project is providing a significantly better understanding of the transport behavior involved in the evaporation process.
During the past year, we focused on two sets of experiments, both of which are designed to help understand the coupling between diffusive and convective transport of vapors from the surface of an evaporating drop. The first set measured the vapor distribution surrounding a methanol drop evaporating in air and the second was the measurement of evaporation rates of hexane and methanol under a wide variety of ambient gas conditions.
Measurement of the Methanol Vapor Distribution
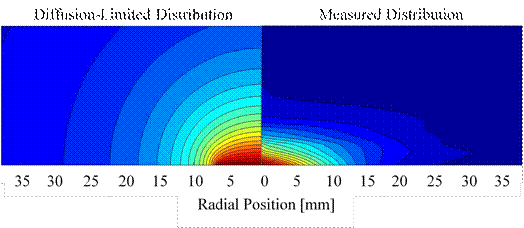
Previously we had measured the vapor distributions surrounding evaporating hexane and 3-methylpentane drops and those distributions dramatically show the strong influence of buoyancy-induced convection. As a follow-up to that work, we chose to investigate the vapor distribution surrounding a methanol drop since it was expected that the influence of buoyancy-induced convection would be negligible due to the fact that the molecular weights of methanol and air are nearly equal. While the measured methanol vapor distribution indicates a greater dependence on diffusive transport than do the hexane and 3-methylpentane distributions, still there are clear differences between the measured distribution and the theoretical distribution for a diffusion-limited case, as shown in Fig. 1, which presents the axially symmetric vapor distributions in a vertical plane passing through the center of the drop.
Figure 1. Comparison of methanol vapor distributions. Left: computed distribution for purely diffusive transport. Right: measured distribution.
In comparison to the diffusion-limited distribution, the measured methanol vapor distribution appears to be pushed down and to become quickly diluted in the radial direction. Those characteristics suggest that some moderate convective flow may be influencing the distribution. Since the mass of methanol is nearly the same as that of air and thus it is unlikely that convection is caused by the density gradient in the vapor-air mixture, we are considering the possibility that evaporative cooling, which may be significant due to methanol’s high heat of vaporization, is chilling the vapor-air mixture near the surface of the drop and thereby causing a thermally-induced natural convection flow. Computed estimates suggest that the vapor-air mixture temperature may drop significantly, however the uncertainty in those calculations is high and measurements of the vapor-air mixture temperature indicate that the temperature drop is small.
To help the analysis of the vapor transport, the distribution of the diffusive flux was calculated for each of the measured vapor distributions and then integrated over various control surfaces surrounding the drop. The total diffusive flux was then compared to the theoretical value for diffusion-limited evaporation, which is the solution to the steady-state Laplace equation. For a control surface near the drop the total diffusive flux is nearly equal to the theoretical diffusive evaporation rate. However, as the cylindrical control surface increases in radius, the diffusive flux increases, which is a consequence of the flattened vapor distribution, and as the cylindrical control surface increases in height, the diffusive flux decreases, which is a consequence of the dilution of the vapor distribution as radius increases. This behavior, which is most pronounced for 3-methylpentane and least pronounced for methanol, is consistent with the hypothesis that the vapor-air mixture flows radially away from the drop over the substrate and causes a downward axial flow of air above the drop.
Measurements under Various Ambient Gas Conditions
The second set of experiments that we conducted during the past year was the measurement of evaporation rates of hexane and methanol in various ambient gas conditions. This study is a follow-up to a previous study in which we correlated the evaporation rates of a wide variety of hydrocarbon drops over a wide range of sizes, but all in room temperature air. That correlation provided some quantitative insight into the nature of the coupling of the diffusive and convective transport. However, the correlation did a poor job of predicting the evaporation rate of hexane under high-pressure conditions or in an ambient gas other than air. Therefore we believe that our correlation is not sensitive enough to the physical processes that vary with ambient pressure and gas. To generate a wide set of conditions, evaporation rate measurements were conducted with hexane and methanol under ambient pressures from 100 to 600 kPa and in the following ambient gases: helium, air, argon, krypton and sulfur hexaflouride. In this way we have conducted experiments for a very wide range of diffusivities and gas densities. Our next task is to attempt to correlate the data with a single equation.
Impact of the Research
This research program has a very large impact on the participating students. During the last year, seven undergraduate students participated in the research and learned valuable lessons not only about the physical phenomena we are studying but, more importantly, about how to go about conducting research, i.e. how to think about problems, how to design and conduct experiments, and how to analyze measurement results. Four of the students devoted 10 weeks during the summer to the research and thereby gained an intensive research experience.
For the first principal investigator, this PRF supported research is his primary research focus and, therefore, is very important for his continuous development as a researcher and academician.
In addition to the direct impact that the project has on students involved and on the first principle investigator, the project is very beneficial to Trinity University’s Engineering Science Department, which is small and completely undergraduate. This PRF-sponsored project has a high profile and supports the largest number of students of the research projects based in Trinity’s Engineering Science Department. Furthermore, the research has nurtured a very productive interdisciplinary collaboration between the principle investigators, one an engineer and the other a chemist and this past year a mathematician has begun collaborating with us.