Reports: UNI753340-UNI7: Tailoring the Chemical Structure of Conducting Polymers and Dopants To Enhance Electromechanical Actuation
Amanda R. Murphy, PhD, Western Washington University
The overall goal of this proposal was to synthesize and evaluate conducting polymer (CP) derivatives that can be systematically varied in order to establish fundamental relationships between the chemical structure of CPs and their ability to undergo electromechanical actuation. In the past reporting year, our efforts have been focused in two main areas: 1) the synthesis, mechanical characterization and actuation performance of crosslinked poly(pyrrole) (PPy) and poly(hydroxymethyl-3,4-ethylenedioxythiophene) (PEDOT-OH) films, and 2) the synthesis of copolymers containing PEDOT-OH and poly(ethyleneglycol) (PEG) segments.
1) Synthesis and Characterization of Crosslinked Polymers.
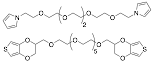
We have successfully synthesized bi-functional monomers capable of forming a cross-linked structure by connecting either pyrrole or (hydroxymethyl-3,4-ethylenedioxythiophene) (EDOT-OH) with a hexaethylene glycol spacer (Figure 1). Different ratios of these bi-functional monomers were then incorporated into PPy and PEDOT-OH films during electropolymerization.
Figure 1. Pyrrole and EDOT-OH crosslinkers.
While the thiophene-based materials have proved troublesome to work with due to the low solubility of the bi-functional monomer and brittleness of the resulting crosslinked films, we have successfully synthesized and characterized a range of pyrrole-based films. As shown in Table 1, optimal mechanical and thermal properties were observed in the resulting films when between 5-10 mol% of the pyrrole crosslinker was added during the pyrrole electropolymerization reaction. Only a slight increase in sheet resistivity was observed at these crosslinking levels, making these intriguing candidates for further investigation.
Table 1. Properties of PPy with increasing amount of crosslinker (mol %) added
|
Film |
% Strain at break |
Ultimate tensile strength (MPa) |
Young's Modulus (MPa) |
Resistivity (Ohms/sq) |
Onset degradation (°C) |
|
Pyrrole only |
3.2 ± 0.2 |
3.76 ± 0.7 |
117 ± 47 |
9.27 ± 1 |
177 |
|
5% Crosslinker |
6.4 ± 1 |
14.9 ±1.4 |
384 ± 83 |
11.7 ± 0.8 |
211 |
|
10% Crosslinker |
9.2 ± 1.5 |
10.48 ± 2.6 |
265 ± 33 |
17.1 ± 2 |
238 |
|
15% Crosslinker |
5.6 ± 0.6 |
8.98 ± 3.7 |
222 ± 78 |
20.5 ± 2 |
198 |
|
25% Crosslinker |
4.8 ± 3.7 |
2.81 ± 0.9 |
89 ± 34 |
51.5 ± 4 |
179 |
The effect of crosslink density on electromechanical actuation was further explored. Bilayer-type actuators were fabricated, submerged in aqueous electrolytes, and cycled between -2 and +2 V while current response and movement were recorded. Similar current responses were observed for actuators made from both plain PPy and 10% crosslinked films. However, a significant downward trend in the extent of movement was observed. Plain PPy showed a maximum deflection of 23° between the fully oxidized and fully reduced states, while the 10% crosslinked films actuated only 13°. The relatively constant current response yet decreased movement is consistent with the fact that the crosslinked films are more rigid. Further work is underway to quantitatively measure the stress and strain generated by these films during actuation using newly acquired instrumentation in our lab (Aurora Scientific Muscle Analyzer). We anticipate submitting a manuscript on this work by the end of the year.
2) Synthesis of Copolymers Containing PEDOT-OH and PEG.
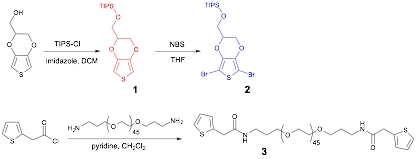
In this reporting year, we have been exploring the use of direct arylation polymerization (DArP) reactions to produce the desired PEDOT-PEG copolymers due to difficulties encountered with the synthetic scheme outlined in our initial proposal. The three monomers needed for DArP were successfully produced as shown in Scheme 1.
Scheme 1. Monomer Synthesis.
For initial polymerization optimization, monomers (1) and (2) were employed. A broad variety of conditions have been explored, and thus far the best results have been obtained using the conditions given in Scheme 2. A purple-red polymer (λmax = 506) with an average molecular weight of 2500 (evaluated with SEC and NMR) was produced, which was soluble in a variety of organic solvents (ex. DCM, THF, CHCl3, toluene).
Scheme 2. Synthesis of PEDOT-OH via Direct Arylation Polymerization.
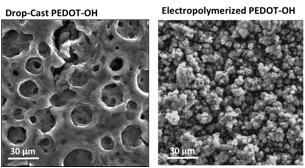
Films were drop-cast onto glass slides, then exposed to a solution of p-toluene sulfonic acid in methanol to remove the TIPS protecting group. Following deprotection, the polymer films were no longer soluble, as is common for PEDOT-OH synthesized via other oxidative methods. As shown in Fig. 2, an interconnected, porous morphology was found for drop-cast films following deprotection, which is distinct from the granular morphology commonly observed in electropolymerized films of PEDOT-OH. The morphology of the drop-cast films is promising for actuation devices as films are anticipated to have improved mechanical properties and electrolyte penetration throughout the porous film. Further evaluation is underway.
Figure 2. SEM image of a drop-cast film of PEDOT-OH.
Using the polymerization conditions described above as a starting point, we are now exploring the synthesis of copolymers that combine monomers (1), (2), and (3) (Scheme 3). Initial trials have shown that (3) can be incorporated into the polymer backbone, but further optimization of the synthesis and isolation procedure is needed. We also plan to vary the molar ratio of (1), (2) and (3) to produce PEDOT-OH-PEG copolymers with different compositions. Further characterization and actuation comparisons of these copolymers will then be performed.
Scheme 3. Synthesis of PEDOT-PEG Copolymer.
Student Impact
Since July 2013, this project has provided research opportunities for 6 undergraduates and one masters student. Ten posters in total have been presented by these students on this work; five at WWU, three at regional symposia (ACS Puget Sound Undergraduate Research Symposium, Pacific Northwest American Vacuum Society Symposium), and two at national meetings (Materials Research Society meeting in San Francisco in April, 2014 and the American Chemical Society meeting in Denver, CO in March 2015). Two students have gone onto master's programs in polymer science (both at U of Oregon), and two more plan to apply to graduate school in 2016 as a result of working on this project.