Reports: DNI354413-DNI3: Development of Sustainable Co(I) Catalyst Toward C-N Bond Coupling
Alison R. Fout, PhD, University of Illinois at Urbana-Champaign
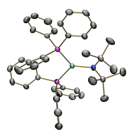
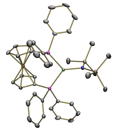
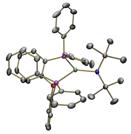
µeff = 3.3 µB Co-CNTMS: Bend: 100 oC µeff = 3.0 µB Co-CNTMS: Bend: 100 oC µeff = 2.9 µB Co-CNTMS: Bend: 100 oC