Reports: UR153237-UR1: Investigation of a Stereoselective Tandem Inverse-Demand Hetero-Diels-Alder/Tin-Free Radical Process for the Synthesis of Highly Substituted Heterocycles
Jake R. Zimmerman, PhD, Ohio Northern University
My research group has benefited tremendously from the support of the ACS-PRF-UR grant. It has allowed me to fund four summer research students during the first two years of the grant and purchase a variety of expendables that were needed for our projects. Two of the students graduated in May and are now attending graduate school. The other students are now in their senior year at ONU and both plan to attend graduate school in chemistry. Because of this support, my group has published two papers and are now very close to another submission. Below is a summary of my group's progress over the past year (9/1/14 – 8/31/15).
My group spent the first half of this grant period working on a project that involved synthesizing a new class of highly fluorescent chromone derivatives. In the first year of the grand period, we discovered an inverse-demand hetero-Diels-Alder (IDHDA) reaction using silylenol ethers and 3-formylchromones. We then synthesized a small library of the Interestingly, it was discovered that these Diels-Alder products were highly fluorescent. Simple irradiation of these compounds using a basic long-wave UV lamp (~365 nm) resulted in blue to green fluorescence (Table 1).
With a series of new fluorophore compounds in hand, absorption and emission maxima along with quantum yields were measured (see Table 1). The enol compounds (1) gave emission maxima from 446-486 nm resulting in blue fluorescence. The sulfonamido-chromone products (2) were a more visibly blue-green to green color of fluorescence with emission maxima ranging from 479 nm to 529 nm. Another general trend observed was that the enol products had much lower quantum yields (ΦF = 2-20% in CH2Cl2) as compared to the sulfonamido-chromone compounds (ΦF= 33-73% in CH2Cl2). Electron-donating groups (EDG's) located on the 6-position of the chromone red-shifted the emission (compare entry 5 with 9, 14, 15 and 16). It was also observed that EDG's increased the quantum yields (for example, compare entry 1 with 3 and entry 5 with 12). A strong EDG on the sulfonamide aryl ring did not affect the emission spectrum or quantum yield (compare entries 16 and 19). It was also observed that the quantum efficiency in both nonpolar and polar solvents was lower for compound 2e (ΦF = 70% in CH2Cl2, 8% in CH3CN and 30% in cyclohexane, entries 9, 10 and 11). A similar trend for 2f was also observed.
Table 1. Absorption, emission and quantum yields for newly synthesized fluorophores.
|
Entry |
Product |
λabsa (nm) |
λemb (nm) |
Stokes Shift |
ΦFc (%) |
|
1 |
1a |
362 |
446 |
84 |
6 |
|
2 |
1b |
364 |
486 |
122 |
2 |
|
3 |
1g |
383 |
482 |
99 |
20 |
|
4 |
1j |
371 |
451 |
80 |
3 |
|
5 |
2a |
358 |
479 |
121 |
37 |
|
6 |
2b |
367 |
492 |
125 |
33 |
|
7 |
2c |
367 |
483 |
116 |
39 |
|
8 |
2d |
343 |
483 |
140 |
34 |
|
9 |
2e |
367 |
492 |
125 |
70 |
|
10 |
2e |
380 |
468 |
88 |
8d |
|
11 |
2e |
364 |
479 |
115 |
27e |
|
12 |
2f |
370 |
490 |
120 |
73 |
|
13 |
2f |
369 |
496 |
127 |
16d |
|
14 |
2f |
364 |
481 |
117 |
30e |
|
15 |
2g |
368 |
490 |
122 |
71 |
|
16 |
2h |
390 |
529 |
139 |
66 |
|
17 |
2i |
358 |
479 |
121 |
38 |
|
18 |
2j |
367 |
492 |
125 |
72 |
|
19 |
2k |
392 |
527 |
135 |
69 |
|
20 |
2l |
386 |
529 |
143 |
67 |
a Absorption maximum. b Emission maximum. c Absolute quantum yields determined by an integrating sphere system in CH2Cl2. dAbsolute quantum yield determined by an integrating sphere system in CH3CN. e Absolute quantum yield determined by an integrating sphere system in cyclohexane.
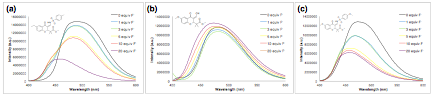
Next, we moved onto a new project that involved using our newly synthesized fluorescent chromones as chemical sensors. In particular, we were interested in the detection of the fluoride anion. The fluoride sensing study began by screening three different compounds from our small library of fluorescent chromone derivatives. Initially we found that enol 4 resulted in nearly no change in fluorescence upon addition of up to 20 equiv. of fluoride (as the tetrabutylammonium—TBA salt, Figure 1(b)). Interestingly, however, sulfinamido-chromone 3 gave a significant decrease in fluorescent intensity (~2.7 fold) and the emission blue-shifted by approximately 33 nm (Figure 1(a)). Chromone 5 bearing two methoxy donating groups gave a similar result (Figure 1(c)), however, it was determined that compound 3 gave a greater fluorescence intensity change (via lowering) and wavelength shift.
Figure 1. (a) Fluorescent spectra of 3 with fluoride equivalents in CH2Cl2 (b) Fluorescent spectra of 4 with fluoride equivalents in CH2Cl2 (c) Fluorescent spectra of 5 with fluoride equivalents in CH2Cl2
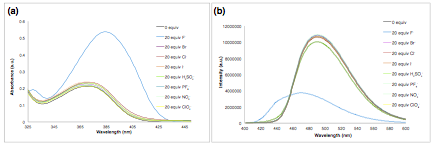
Based on the results from Figure 1 and other data not shown, it was determined that of sulfonamido chromone 3 was the optimal sensor at this point in time. Therefore, we moved forward with 3 and studied the selectivity for fluoride with respect to other anions. The UV-vis absorption and fluorescence spectra were conducted in the presence of 20 equiv. of several common anions as their tetrabutylammonium (TBA) salts. In the absence of the anions, sulfonamide 3 shows UV-vis λmax of 370 nm in CH2Cl2. The absorbance of sulfonamido chromone ([3] = 5.0 x 10-6 M in CH2Cl2) was red–shifted to λmax = 386 nm (Δλ = 16 nm) upon addition of 20 equiv. of fluoride anions as depicted in Figure 2(a). There was no notable change of the absorbance spectrum of 3 in the presence of other anions. As shown in Figure 2(b) and Figure 3, the presence of fluoride was easily detected by the change in emission color and intensity of 3. It was also observed that there was no noticeable change in color or intensity upon addition of other anions.
Figure 2. (a) Absorbance and (b) Emission spectra of 5.0 μM 3 in the presence of various common anions (as TBA salts).
Figure 3. Vials of 0.25 mM 3 with 20 equiv. of anions. From left to right: no anion, F-, Br-, Cl-, I-, HSO4-, PF6-, NO3-, ClO4-.
In conclusion, we will continue to investigate the synthesis and sensing properties of these new fluorescent chromones.