Reports: DNI654668-DNI6: Fundamental Investigations of Plasmons and Electron Dynamics in Petroleum-Derived Polycyclic Aromatic Hydrocarbons
A. Eugene DePrince, Florida State University
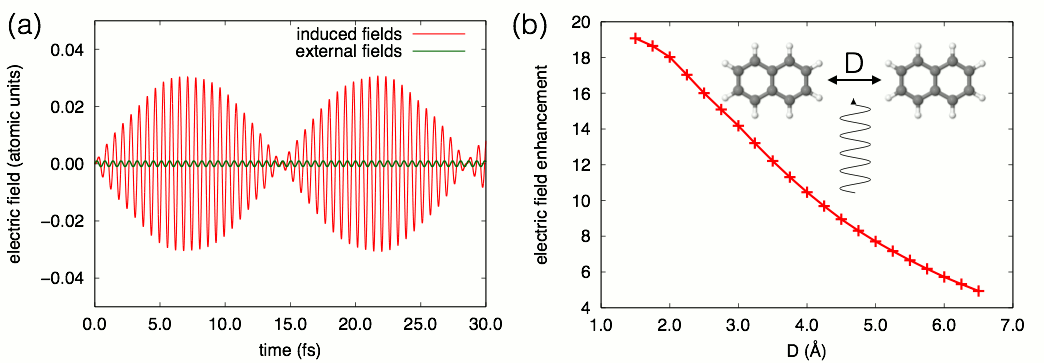
Figure 1. A resonant external electric field induces Rabi oscillations in
a naphthalene dimer.