Reports: UR152067-UR1: The Characterization and Synthetic Utility of Complexes Made from Samarium Diiodide and Anionic Phosphoramides
Chriss E. McDonald, PhD, Lycoming College
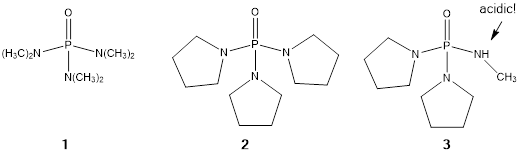
Samarium diiodide is one of the most useful reagents employed by organic chemists. The utility of this reagent is greatly enhanced by the addition of electron rich cosolvents. HMPA (1, Scheme 1) is the most common cosolvent to be employed and the majority of modern SmI2 chemistry is accomplished with this cosolvent (Scheme 1). Unfortunately HMPA is a mutagen which has leaded to an extended search for suitable alternatives. The goals of this work are to find SmI2 activators which are both safer and more effective. We have recently reported that TPPA (2) is approximately an order of magnitude better at activating SmI2 at two of its key synthetic processes, reduction of carbon-halogen bonds and reduction of ketones. Our most recent published work involves the use of deprotonatable phosphoramides such as DPMPA (3). Complexes formed from SmI2 and four equivalents of the anion derived from deprotonation of 3 (DPMPA-) proved to be > 100X more reactive than the SmI2/HMPA complex as determined by the reduction of 1-chlorodecane.
Scheme 1. Phosphoramides.
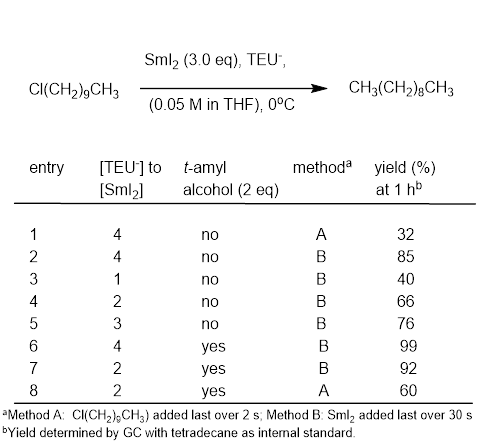
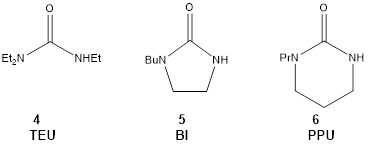
Recently we have focused on extending this approach to ureas. Advantages of ureas over phosphoramides include lack of mutagenicity, lower molecular weight, and ease of synthesis. Due to the lower valency of the central atom, the conjugate bases of ureas will potentially form less sterically encumbered complexes with SmI2. The most common urea used in synthesis, DMPU, is known to be a modest activator for SmI2. After a series of screening reductions of 1-chlorodecane, we have decided to focus on three deprotonatable ureas as shown in Scheme 2.
Scheme 2. Deprotonatable Ureas.
Triethylurea (TEU, 4) was selected for its
demonstrated ability to activate SmI2 for R-X reduction as well as
its ease and low cost of synthesis. N-Butylimidazolidinone (BI, 5)
and N-propylpropylene urea (PPU, 6) are better activators than
TEU but made from more costly starting materials. Because of the centrality of
TEU to our program, we engaged in an optimization study for the reduction of
1-chlordecane (Table 1). It was determined that the order of addition
mattered greatly. Adding SmI2 last (Entry 2, Method B) works much
better than adding 1-chlorodecane last (Entry 1, Method A). Although the
reaction proceeds without a proton source, the addition of t-amyl
alcohol does increase the yield. Interestingly, even two equivalents of TEU-
per equivalent of SmI2 in the presence of t-amyl
alcohol is sufficient for near quantitative reduction of 1-chlorodecane in 1
hour (Table 1, Entry 7).
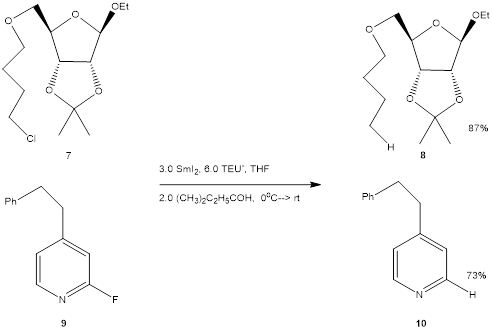
We used this newly optimized procedure (analogous to Entry 7
in Table 1) to reduce several alkyl chlorides and even fluorides.
Chlororiboside 7 and pyridyl fluoride 9 are illustrative (Scheme
3).
Scheme 3. Selected R-X Reductions.
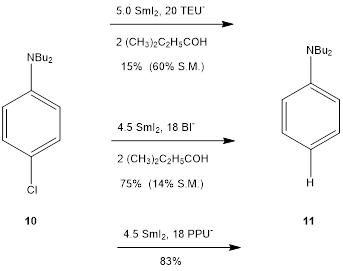
With less reactive alkyl and aryl halides the better
activators BI- and PPU- must be employed (Scheme 4).
In this case the strongly electron donating amino substituents slows the
initial reduction necessitating the use of stronger activators for
synthetically useful reactions.
Scheme 4. The reduction of 4-N,N-Dibutyl-1-chlorobenzene.
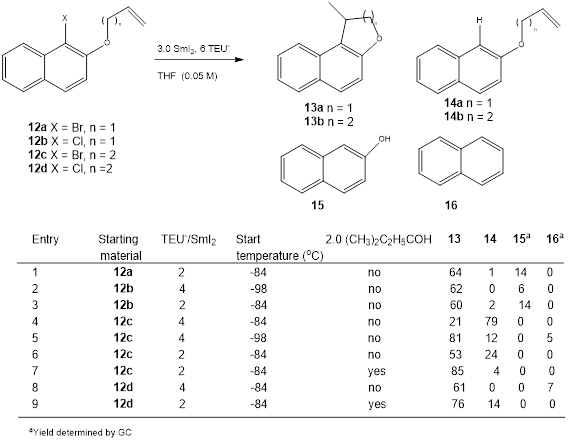
We are also interested in the ability of our SmI2/TEU-
complexes to effect radical cyclization of appropriately substituted haloalkenes.
A series of naphthyl-based substrates were cyclized (Table 2). Both the
2:1 and 4:1 complexes were employed. The proton source t-amyl alcohol
was also used with the 2:1 complex. Side reactions include premature reduction
to afford reduced uncyclized products 14a and b, reductive
deallylation of the O-allyl species leading to Table 2. Cyclization of Haloalkenylnaphthalenes.
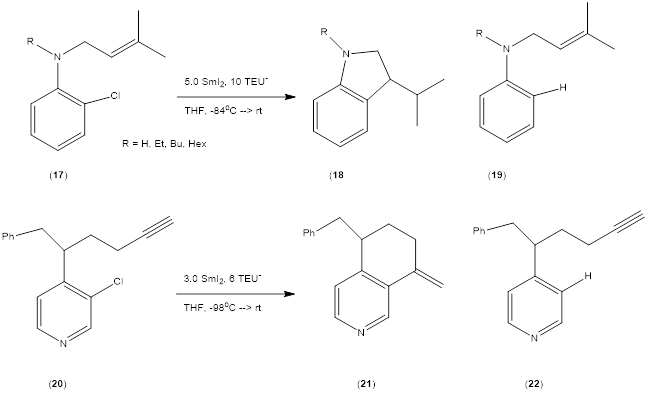
We are also involved in radical cyclizations of aniline and
pyridine-based systems. The two substrates shown in Scheme 6 are
typical. Substrate 12 has a substantial tendency to afford the reduced
uncyclized product 14 when R is H or Et. With the larger Bu and Hex
substituents the cyclization product 13 is favored.
Scheme 5. Other radical cyclizations.
Current work in this laboratory focuses on the use of other
reluctant radical precursors, as well as the development of new SmI2
reactions.