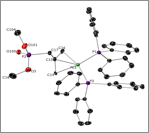
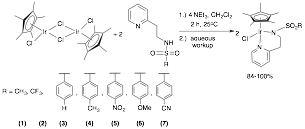
Reports: UNI353605-UNI3: Synthesis of Cationic Nickel(II) Complexes Containing Hemilabile Groups for Use as Alkene Hydrogenation Catalysts
Abby R. O'Connor, PhD, The College of New Jersey
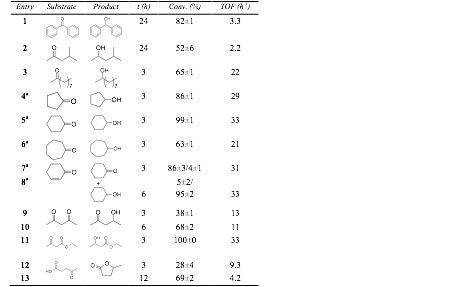
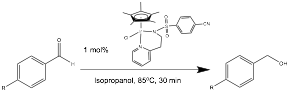
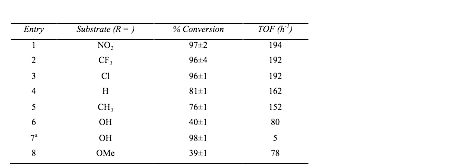
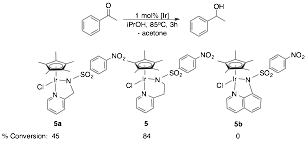
1H NMR spectroscopy used
to determine yield. Yields reported as an average of 3 trials.
1,4-Dimethoxybenzene used as standard. 1.0 M solution of substrate in
isopropanol.
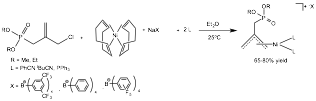
![]()