Reports: UR451689-UR4: Exploring the Controllable Uptake and Release of Pyridinium Ions with a Photoactive Calixarene-Capped Azobenzene
Paul A. Bonvallet, PhD, College of Wooster
I. Technical progress
Overarching Goal
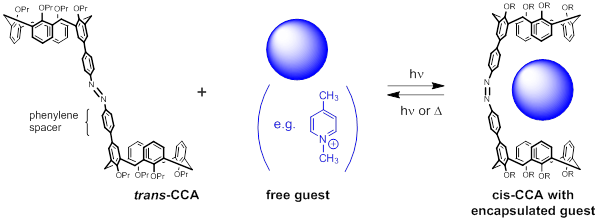
We are developing a molecular capsule for the controllable uptake, storage, and release of cationic guests. By virtue of the convergent calix[4]arene hemispheres connected by a photoactive azobenzene bridge, we anticipate that this calixarene-capped azobenzene (CCA) host will be able to selectively entrap various pyridinium-, ammonium-, and diimidazolium-derived guest species shown below. The function of this light-activated capsule comes from the trans-cis photoisomerization reaction of azobenzene, whereby two geometrically distinct “open” and “closed” forms can be produced in response to light.
Progress over the Past Year
Following an exhaustive survey of 13+ candidate cationic molecules, we have yet to identify a guest that binds more strongly to the cis CCA host than to the trans one. These challenges originate from two properties of the host:
1) CCA is very nonpolar, making it insoluble in most solvents that would be used to dissolve a cationic guest
2) The two phenylene spacers create a cavity that is fairly large relative to the size and aspect ratio of the guests. We believe that guests bind only to one hemisphere of CCA, irrespective of whether the host is in the cis or trans configuration.
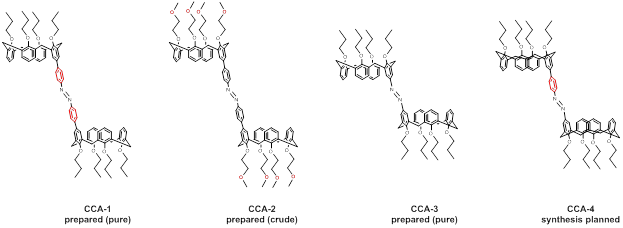
We therefore have focused our efforts on redesigning a group of next-generation hosts, pictured below.
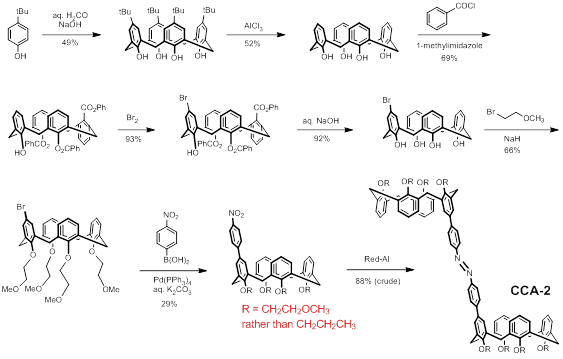
In CCA-2, the propoxy groups along the calixarene “lower rim” have been replaced with 2-methoxyethoxy groups (Scheme 1). This modification is predicted to enhance the solubility of the host in polar organic solvents and thereby make both host and guest species mutually soluble in the same solvent. Control experiments with simpler calix[4]arene model compounds demonstrate a 100-fold increase in solubility in acetonitrile when the lower rim is substituted with 2-methoxyethoxy groups rather than propoxy groups. With CCA-3 we envision the removal of both phenylene spacers so as enhance the strength of binding with guests by shrinking the size of the cavity in the cis host (Scheme 2). The synthetic schemes for these two compounds are shown below.
Scheme 1: Preparation of a More Soluble Host
Scheme 2: Preparation of a Small-Cavity Host
We have data on a crude sample of CCA-2 that indicates the successful preparation of this compound. Purification and host-guest studies with this next-generation material are forthcoming. CCA-3 has been synthesized and fully characterized. Interestingly, CCA-3 does not undergo the photochemically driven trans/cis isomerization that is characteristic of azobenzene derivatives. It is inert under UV irradiation and, after prolonged exposure, gradually decomposes. This surprising lack of reactivity is novel and will be explored further. Part of this investigation involves the synthesis of CCA-4 (having one phenylene spacer along the azobenzene unit, rather than two or zero) to determine the influence of these spacers on photochemical activity.
II. Impact on undergraduate development
Synthetic progress towards CCA-2 was the Senior Independent Study research project for Virginia Iungerich during the 2014-2015 academic year. She was supported as a summer researcher by this grant for both the summer of 2013 and 2014. Virginia graduated with a chemistry degree in May and is currently employed as a chemist at VideoJet Technologies in the Chicago area.
Additionally, two students were directly supported during the summer of 2015: Albert Darling (senior chemistry major) and Charles VanDenbergh (sophomore). Albert is planning to attend graduate school in chemistry, while Charles is likely going to declare a major in chemistry this year. They worked on the preparation of CCA-2 and CCA-3, respectively, and presented their results at a poster session during family weekend at the College of Wooster in September 2015.
I presented the progress of all three students’ efforts at the Spring 2015 and Fall 2015 national meetings of the American Chemical Society. Virginia traveled, with PRF support, to Vancouver in November 2014 to present her research at the North American meeting of the Society of Environmental Toxicology and Chemistry (SETAC). Her emphasis during that conference was on the potential for CCA compounds in environmental remediation. She also presented at the day-long Senior Independent Study showcase held on campus every April.
This group of curious and dedicated students benefitted tremendously from PRF support. The senior students (Virginia and Albert) had an opportunity to develop deep expertise in a focused area, while Charles got to undertake organic synthesis for the first time ever. They became experts in experimental design and instrumental methods in organic chemistry. In terms of process-oriented skills, these researchers developed independence and creative problem solving. We had a community of students during the 2015 summer research season who regularly met and formally communicated their results to their peers. Thanks to the support of PRF, these students could be scientists and think like a practitioner in the field.
III. Impact on PI development
This project supported by PRF is one of the staples of my Senior Independent Study and summer research with students at the College of Wooster. Over the past year I presented their research at two separate national meeting of the ACS. During the Boston meeting (August 2015) I was an invited speaker for the “Small Splash, Big Waves” symposium, a session dedicated entirely to undergraduate research in organic chemistry that is conducted at PUIs.
Travel support continues to be invaluable. While at the Denver ACS meeting (March 2015) I met with Professor Xiaopeng Li from Texas State University San Marcos to discuss ideas for our growing collaboration. The synthetic expertise from my laboratory enabled us to contribute various calix[4]arene building blocks for his research on self-assembled metallo-supramolecular scaffolds. Dr. Li is an expert in mass spectrometry and has undertaken a few experiments in which trans and cis CCA compounds can be differentiated – even with the same m/z ratio – according to their shape via ion mobility measurements. The support from PRF has enabled me to raise the visibility of my research, nurture collaborations, and continue contributing to the scientific development of undergraduate students.