Reports: DNI1054544-DNI10: Investigating Polymer Templated Graphene Scaffold for Heterogeneous Gold Nanoparticle Catalyst in Selective Oxidation Reactions
Yu Zhu, PhD, University of Akron
Introduction
Fossil fuels are major sources of the world's organic building blocks. In order to obtain various useful intermediates for chemical industry, these simple hydrocarbons have to be partially oxidized rather than completely burned to carbon dioxide. Selective oxidation catalysts play important roles in such processes. The central thesis of this research is to synthesize and characterize novel heterogeneous catalysts with ultrasmall gold nanoparticles (NPs) on organized graphene scaffolds for selective oxidations. In his research, graphene serves as a two-dimensional multifunctional substrate, which not only provides mechanical scaffolds for small gold NPs, but also guides the formation of nanoparticles to obtain well-organized, uniform nanoparticle arrays. Such a novel hybrid system may provide new opportunities to develop selective oxidation reaction catalysts.
The major goals during this reporting period were (1) to synthesize ordered 3-dimensional porous high quality graphene that readily serve as the substrate of catalysts and (2) to synthesize gold nanoparticles for integrating with the graphene scaffolds.
Accomplishments
Synthesis of 3D graphene scaffolds
In the past year, we demonstrated a novel method to fabricate 3D bi-continuous graphene by using polymer templates. The fabrication procedure of graphene monolith is illustrated in Figure 1: First, polystyrene (PS) and polyethylene oxide (PEO) powders were dry-mixed with a twin-screw extruder to form a bi-continuous polymer blend. Second, the polymer blend was treated with water to remove the polyethylene oxide. The resulting PS template was coated with Ni by electroless deposition. Following this step, the PS was dissolved in toluene and the free-standing Ni template was formed. Finally, the graphene was grown on the Ni template by chemical vapor deposition. A free-standing, bi-continuous, 3D graphene monolith was obtained after removing the Ni scaffold.
Figure 1 Schematic illustration of graphene monolith preparation.
This method allows for the fast fabrication of large graphene scaffold samples with good yield. Figure 2 shows optical images of templates and porous materials prepared in this procedure. The polymer blends were compressed into disks as shown in Fig. 2a. This pale-yellow sample was treated with water to remove the PEO and obtain the white PS disk sample in Fig. 2b. The PS disk sample was coated with Ni by electroless deposition and then PS was removed by organic solvent leaching, resulting in the black Ni disk sample in Fig. 2c. The Ni disk was cut into smaller pieces (Fig. 2d) in order to fit the size of CVD tube furnace. The graphene covered Ni template following CVD growth is shown in Fig. 2e. Finally, the Ni was removed. The resulting light-weight graphene monolith is shown in Fig. 2f.
Figure 2 a) Optical image of polymer blends (PS/PEO) film. b) PS film after removing PEO. c) Electroless Ni template. d) A small Ni sample from (c) that fits the size of CVD furnace. e) The graphene covered Ni template after CVD growth. f) The free-standing porous graphene sample in a glass vial.
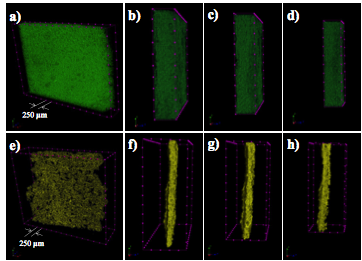
We carefully characterized the 3D bi-continuous structure obtained from this process by X-ray microcomputed tomography (µCT). In this study, the sample was mounted on the rotating stage with a micro-focus X-ray source. The projection images were captured by a planar multichannel x-ray detector. From these 2D shadow projection images, the structures of the templates were reconstructed. The reconstructed images are shown in Figure 3. Figures 3a-3d show the reconstructed PS templates. The porosity of the PS template is 62.1%. Figures 3e-h are reconstructed 3D graphene covered Ni. The porosity of sample is 94.0%.
Figure 3 X-ray tomography of bi-continuous templates a-d) PS template e-h) graphene covered nickel template.
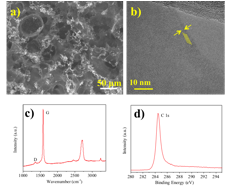
Free-standing porous graphene was further characterized by a suit of tools including Raman, XPS, TEM and SEM. As shown in Figure 4, the free standing graphene has porous structure and the typically contains 3-5 layers. The Raman and XPS spectra indicated the graphene has very high quality.
Figure 4 The characterization of bi-continuous 3D graphene a) SEM image of graphene b) TEM image of graphene c) Raman spectrum of graphene and d) High-resolution XPS C1s spectrum of graphene.
Synthesis of Gold Nanoparticles
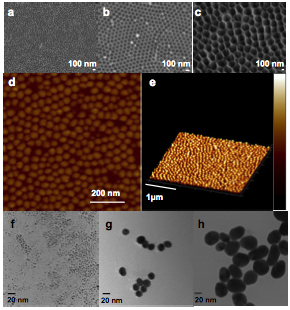
Two methods have been investigated this year to fabricate gold NPs. In the first method, an anodic alumina (AAO) shadow mask was prepared and used in the ebeam evaporation process to generate uniform gold NPs. Figure 5 a-c show the SEM images of AAO membrane. The channel length of the AAO template is ~ 500 nm, therefore, the gold atoms can penetrate the membrane and deposit on the surface uniformly. We have demonstrated the gold nanoparticles arrays with diameter around 50 nm could be prepared (Fig. 5d,e). The optimization of this experiment is still on-going to obtain smaller gold NPs. In the second method, we synthesized small gold cluster through wet chemistry. The gold atoms nucleated in solution and form uniform gold NPs with size from ~ 3 nm to 80 nm. (Fig. 5 f-h)
Figure 5 Synthesis of gold nanoparticles a-c) SEM of AAO membrane with different pore size a) 40 nm, b) 80 nm c) 300 nm. d-e) AFM images of gold NPs deposited by AAO membrane e-g) TEM images of gold NPs prepared from wet chemistry method with different size e) 3 nm f) 20 nm g) 50 nm.
Educational efforts from this research
Graduate students funded by the ACS PRF grant fabricated graphene 3D scaffolds by polymer blends templates. They also worked together with a self-supported master student to develop the methods to synthesize small gold nanoparticles. Through this study, they received a broad training experience, acquired fundamental knowledge of polymer blends, chemical vapor deposition, nanoparticle synthesis and a suit of fabrication/characterization tools such as e-beam evaporation and transmission electron microscope. They also assisted in manuscript preparation and presented their work at departmental seminar.
Future Plan
In the following year, we will integrate the nanoparticles with porous graphene scaffolds to assemble the heterogeneous catalyst. We will then conduct the oxidation experiments to evaluate the catalytic behavior of those novel catalysts.