Reports: DNI653105-DNI6: Experimental Investigations of Radical-Particle Reactions Relevant to Hydrocarbon Pyrolysis
Fabien Goulay, West Virginia University
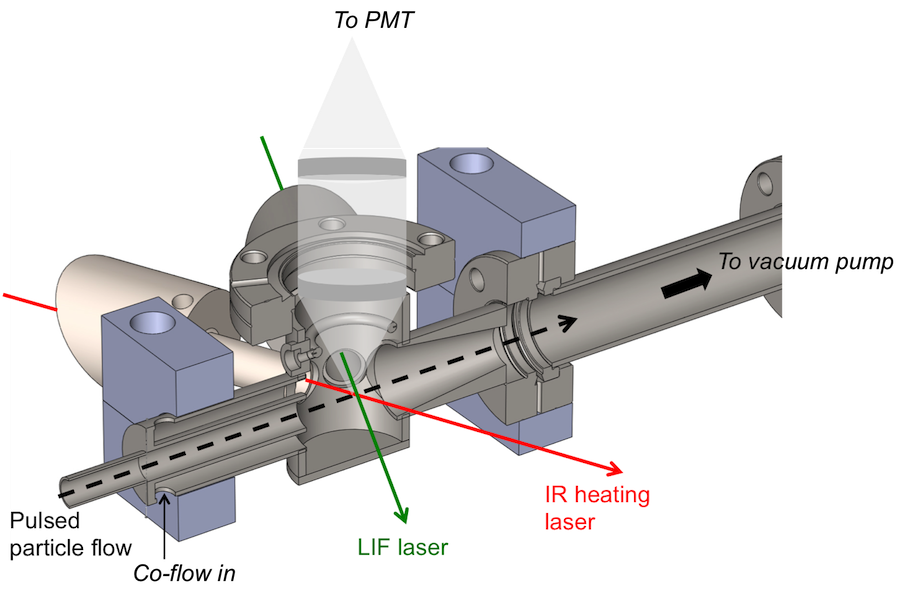
Experimental
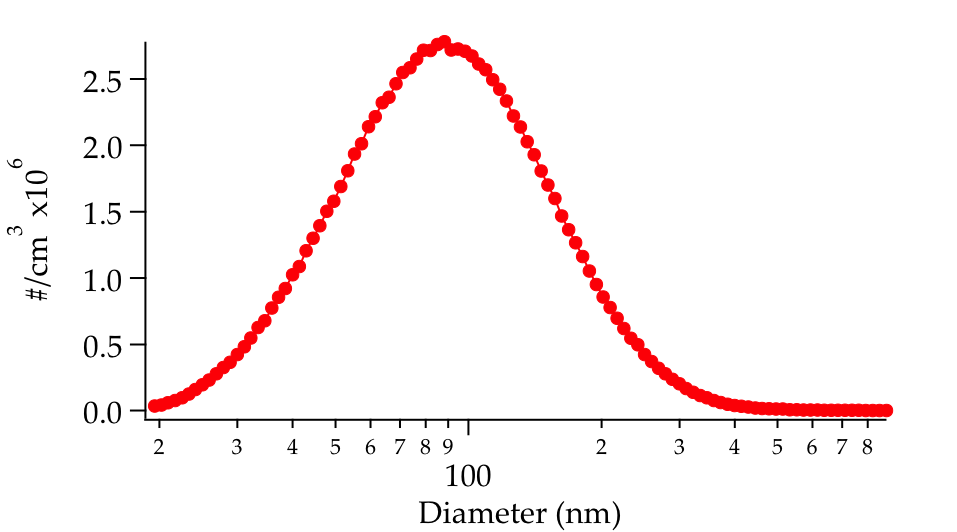
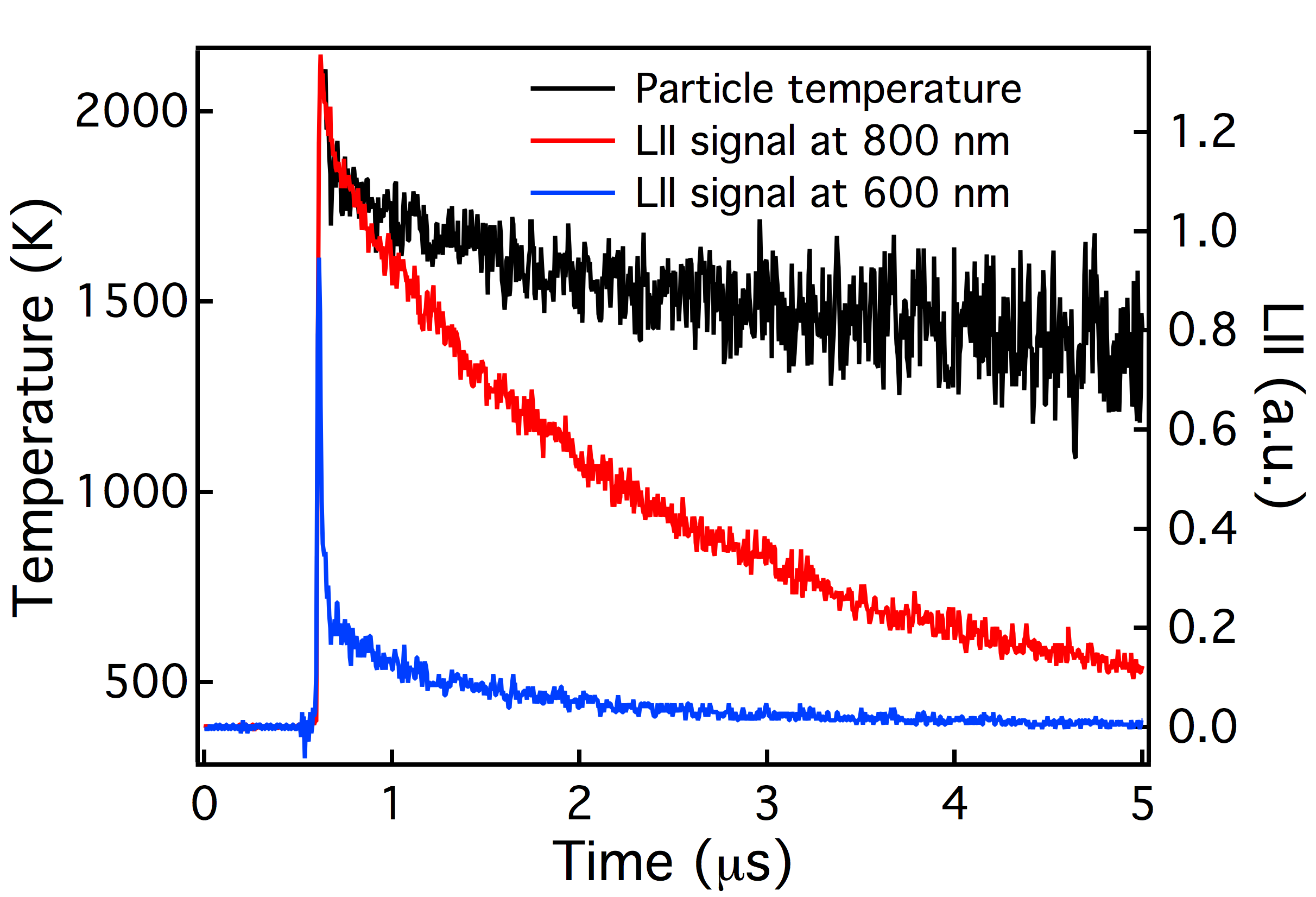
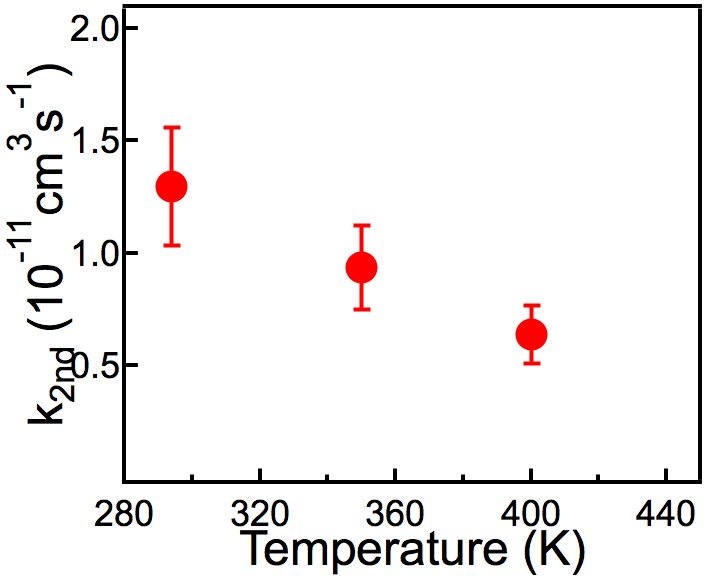
Heterogeneous reactions of the OH radical
Gas phase kinetics of soot precursor formation