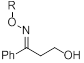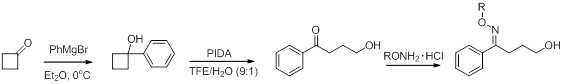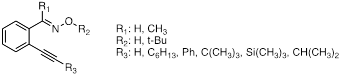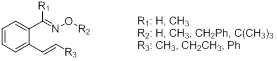Reports: UR453793-UR4: Mechanistic Studies of Radical and Radical Ion Intermediates in Oxidative Processes
H. J. Peter de Lijser, PhD, California State University (Fullerton)
Research in our group focuses on the electron transfer chemistry of the carbon-nitrogen double bond. Of particular interest are oximes and oxime ethers because of their stability (compared to imines), their use as protection groups in organic synthesis, and the increasing use of these compounds in pharmaceuticals. Photooxidation or enzymatic oxidation may result in the formation of reactive species, such as radical ions or free radicals, which can cause damage to cells and tissue in biological systems. Our goal is to investigate the oxidative chemistry of oximes and related species and to obtain a complete understanding of the involvement of reactive intermediates in these processes.
In recent years we have shown oxidation of oximes leads to iminoxyl radicals as intermediates whereas oxidation of oxime ethers produces oxime ether radical cations. The different chemistry observed with these different species is being explored for synthetic utilization. Of particular interest is to see whether we can perform intramolecular cyclization reactions to generate interesting heterocyclic structures. To do so we are exploring the intramolecular trapping of the radical and radical ion species with built-in alcohol groups, aromatic rings, alkynes, alkenes and cyclopropyl rings. Progress has been made in several areas.
One project has focused on the use of built-in alcohol groups as the nucleophile (Figure 1). The synthesis of the desired compounds from has proven to be significantly more challenging than originally anticipated but in the past year we have succeeded in our goal to prepare g-hydroxy-1-phenylbutanone oxime ethers (Scheme 1). The substrate was studied in a variety of solvents and with different sensitizers. On the basis of NMR analysis, a cyclization reaction seemed to occur, however, further analysis of the reaction mixture suggested the formation of a different product, which has not yet been positively identified.
Figure 1. Set of hydroxyoxime ethers used for studies of intramolecular cyclization reactions of oxime ether radical cations.
Scheme 1. Synthetic scheme for the preparation of g-hydroxy-1-phenyl-1-butanone oxime ethers.
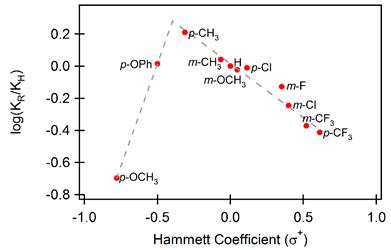
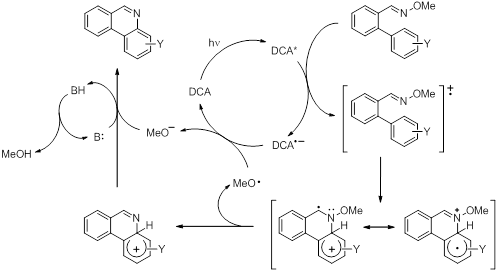
We have previously shown that aromatic rings act as nucleophiles for attack on oxime ether radical cations). Under the conditions of the reaction, the photoinduced electron transfer (PET) process was found to be catalytic in nature and good yields and regioselectivity of phenanthridines could be obtained when using substituted aromatic rings. Mechanistic studies showed an interesting observation; a Hammett analysis revealed change in the rate-limiting step when more powerful electron-donating substituents were present on the aromatic ring. This was explained in terms of a change in oxidation site, which affects the rate-limiting step of the overall sequence (Figure 2, Scheme 2). To build on our initial work we have set out to investigate the oxidative cyclization reactions of heteroatom containing aromatic rings in order to build ring structures with multiple heteroatoms. The synthesis of these compounds was found to be more challenging although 8 different compounds have been made so far. Under the conditions of the previous reactions, no cyclization was found to occur, something that can possibly be attributed to the fact that these compounds are similar to compounds containing strongly electron donating substituents and oxidation may have shifted to a different part of the molecule. Electrochemical measurements and computational chemistry will be done in the near future to shed more light on this.
Figure 2. Hammett plot of PET cyclization reaction of substituted 2’-arylbenzaldehyde oxime ethers.
Scheme 2. Proposed mechanism for the formation of phenanthridines from the 2’-arylbenzaldehyde O-methyl oxime ether radical cation intermediate.
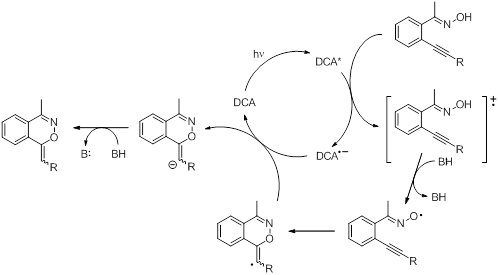
Much of our work in this particular area has been done using a series of o-alkynylacetophenone and benzaldehyde oximes and oxime ethers (Figure 3). To learn about the alkyne moiety and its behavior as a radical trap or a nucleophile, a variety of different alkyne containing substrates (oximes and oxime ethers) were prepared and studied. Both photochemical and thermal oxidations were carried out and the reactions were followed by GC/MS and NMR. Cyclization is believed to occur in several cases. More interestingly, the cyclization seems to be an exo-type cyclization, which has not been observed for these types of species. So far all our data is based on NMR experiments; isolation of the products has been challenging because of thermal instability of the proposed product. Much time has been spent on preparing the desired materials again and to repeat all our previous work on these compounds (carried out independently by a different student). The most obvious evidence for the cyclization pathways comes from the observed splitting patterns in the NMR spectra of the substrates where R3 = n-hexyl (triplet), isopropyl (doublet), and t-butyl (singlet). A variety of other experiments have been carried out and the mechanism seems to be most consistent with formation of the iminoxyl radical, followed by cyclization onto the alkyne moiety and hydrogen atom abstraction to produce the final product (Scheme 3). Further work will focus on isolating or trapping the proposed intermediate as well as computational studies to study the energetics of these cyclization pathways, the intermediates involved and the final products.
Figure 3. Set of o-alkynylaryl oximes and oxime ethers used for studies of intramolecular cyclization reactions of iminoxyl radicals and oxime ether radical cations.
Scheme 3. Proposed mechanism for the catalytic oxidative cyclization of o-alkynylaryl oximes.
Since the alkyne and aromatic substrates discussed above seem to react differently, we have recently set out to prepare a set of alkene-containing substrates (Figure 4) to determine their reactivity; it is possible that these substrates will display features of both sets. The synthesis of these substrates has been difficult and little progress has been made so far. A stilbene derivate was prepared (R1 = H; R2 = H, CH3; R3 = Ph), however it was shown to absorb light (even at 420 nm), which made its reactions undesirable. Other methodologies for the preparation of substrates of interest are now being considered.
Figure 4. Set of o-alkenylaryl oxime ether targets to be used for studies of intramolecular cyclization reactions of iminoxyl radicals and oxime ether radical cations.