Reports: UNI354044-UNI3: Preparation, Electronic Structure, and Reactivity Studies of Iron Complexes Supported by Conjugated Alpha-Diimine Ligands
Helen Hoyt, PhD, Knox College
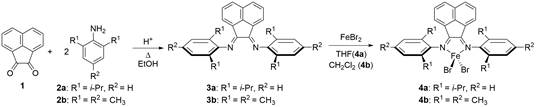
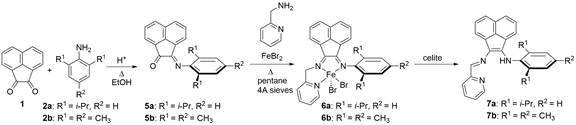
Helen Hoyt, PhD, Knox College


Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society