Reports: DNI1052877-DNI10: Improving the Cyclability of Iron Pyrite (p-FeS2) Cathodes in Lithium Ion Batteries Through Carbon Nanotube Encapsulation
Yongan Yang, PhD, Colorado School of Mines
1. Statement of the Problem:
The development of iron pyrite (p-FeS2) cathode for lithium ion batteries (LIBs) at room temperature is attractive. The key challenge is how to achieve high cyclability, for which the impeding factors have typically been ascribed to: 1) the poor electrical conductivity of the discharge product, Li2S; 2) irreversible chemical reactions between p-FeS2 and the electrolyte; 3) the loss of materials due to the formation of soluble polysulfides; and 4) the partial loss of the electrical contact between p-FeS2 and the current collector due to the volume-fluctuation during the discharging/charging cycles.
2. Goal of the Grant:
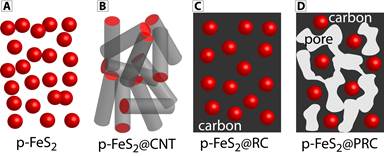
Our goal is to develop an efficient mitigation strategy to improve the cyclability of iron pyrite (p-FeS2) cathodes through carbon encapsulation (Figure 1).
Figure 1. Schematic illustration of the electrode materials to be studied in this project. (A) The as-synthesized p-FeS2 nanocrystals; (B) Carbon nanotube encapsulated p-FeS2, p-FeS2@CNT; (C) Resilient carbon encapsulated p-FeS2, p-FeS2@RC; and (D) Porous resilient carbon encapsulated p-FeS2, p-FeS2@PRC.
3. Research Progress:
3.1 Cyclability of the four types of p-FeS2 electrodes
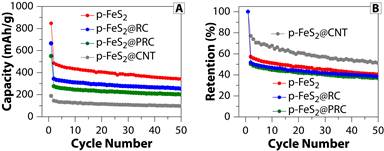
Figure 2 shows the cyclability (A) and the corresponding retention profiles (B) of four types of p-FeS2 electrodes illustrated in Figure 1. Evidently, the bare p-FeS2 electrode shows the best performance. Its initial capacity is 850 mAh/g, well consistent with the theoretical value 890 mAh/g. In the second cycle, the capacity drops to 500 mAh/g, 59% of the initial value, close to the theoretical value 69%. After 50 cycles, the capacity keeps at 340 mAh/g. In contrast (Figure 2B), the electrode seems to show the best retention, while other three present comparable retention profiles. These results imply that the synthesized p-FeS2 behave well intrinsically and the carbon encapsulation (CNT, RC and PRC) could not keep good p-FeS2/C interfaces during the first cycle, probably due to the mechanical damage and/or poor conductivity of the carbon matrix.
Figure 2. (A) Cyclability for the four types of electrodes: p-FeS2 (red), p-FeS2@CNT (gray); p-FeS2@RC (blue), and p-FeS2@PRC (green); (B) The corresponding retention profiles. The charging/discharging rate is 0.05 C (1C = 890 mA/g).
3.2 Rate capability of the p-FeS2 and p-FeS2@RC electrodes
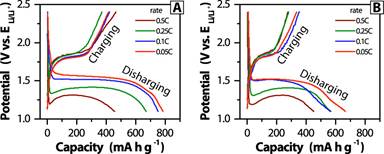
The p-FeS2 and p-FeS2@RC electrodes are chosen for the further study of rate capability (Figure 3) using the charging/discharging rates of 0.05 C - 0.5 C. These two electrodes exhibit some similar behaviors: the charging and discharging plateau are at 1.75 V and 1.25 - 1.53 V, respectively; the capacity and discharging plateau decrease with increasing the rate.
Figure 3. Potential profiles against the specific capacity of p-FeS2 (A) and p-FeS2@RC (B) at four charging/discharging rates
3.3 Synthesis of p-FeS2 nanocrystals using different solvents
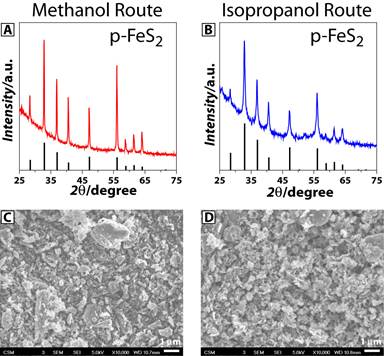
By analyzing Figure 2 and Figure 3, we wonder if the performance can be improved by changing the solvent used for materials synthesis. Figure 4 shows the X-ray diffraction (XRD) and scanning electron microscope (SEM) characterization of the p-FeS2 nanocrystals synthesized by changing the solvent from ethanol to methanol (A and C) and isopropanol (B and D). The XRD results indicate that the methanol-based p-FeS2 nanocrystals have larger crystalline sizes (35 nm vs 18 nm), while SEM images show similar sizes (~100 nm) of the secondary particles (aggregated individual crystals) and morphology. Note that, compared with the methanol-based p-FeS2 nanocrystals, the ethanol based p-FeS2 nanocrystals have similar crystal sizes but larger secondary particles (~500 nm).
Figure 4. XRD (A and B) and SEM (C and D) characterization of the p-FeS2 nanocrystals synthesized by using different solvents of methanol (A and C) and isopropanol (B and D).
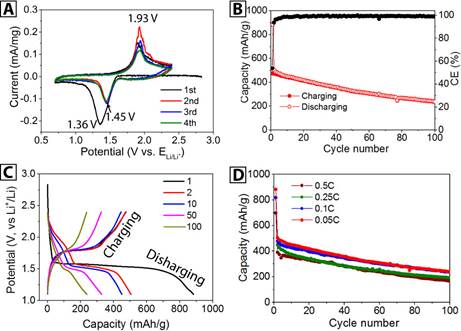
3.4 Systematic study of the methanol-based p-FeS2 nanocrystals
Figure 5 shows the systematic study of the p-FeS2 nanocrystals synthesized via the methanol route. The cyclic voltammograms (Figure 5B) are similar to those of ethanol based p-FeS2 nanocrystals; the initial cathodic peak at a lower potential (1.36 V) can be ascribed to need of overcoming an activation barrier of the lithiation process. The cyclability is also very similar to that of ethanol based p-FeS2 nanocrystals, that is, the capacity at the 1st, 2nd, and 50th cycles is 880 mAh/g, 500 mAh/g, and 325 mAh/g, respectively. The coulombic efficiency is also similar. The potential profiles (Figure 5C) present the typical potential plateaus and degradation trend of p-FeS2. The initial capacity at different rates (Figure 5D) are higher than those of ethanol based (Figure 3A), while the retention profiles are similar.
Figure 5. Systematic study of the methanol based p-FeS2 nanocrystals. (A) The first four cycles of cyclic voltammograms; (B) The cyclability (red) and coulombic efficiency (black); (C) Several selected potential profiles against the capacity; and (D) Cyclability at four charging/discharging rates.
4. Broader Impacts:
This project in the past one year has resulted in multi-faceted impacts on all participants. We believe these impacts will continue to manifest in our future research and life.
The PI has improved his scholarship in many aspects, including the tactics for approaching scientific problems, the skills of mentoring graduate/undergraduate students, the capability of developing new projects, and national/international recognition. This grant has generated two publications on high-impact journals (Chemistry of Materials, 2014, 26, 6743–6751; IF 8.4); Journal of Power Sources, 2015, 274, 685–692; IF 6.2). One new manuscript is in preparation.
Three female graduate students (Tara Yoder, Jacqueline Cloud, and Xuemin Li) have been partially supported by this grant. Their passion and competency in scientific research have been greatly enhanced. Yoder and Cloud have graduated with PhD degrees in Dec. 2014. Tara, with waived registration fees, presented our work at the 226th ECS meeting in Cancun, Mexico during Oct. 5-9, 2014. Cloud has been inspired to continue her academic career as a postdoctoral researcher at University of Connecticut. Xuemin has been well trained in electrochemistry and battery assessment.
One female undergraduate student, Ms. Lauren Cain, was also attracted to participate in this grant. She has collected valuable data and will coauthor on a new manuscript in preparation.
Our collaboration with two colleagues in our department and one colleague at UCLA has been greatly strengthened through this grant.