Reports: ND1054793-ND10: Study of Transition Metal Chalcogenide Nanostructures as Efficient Oxygen Reduction Catalysts
Manashi Nath, PhD, Missouri University of Science and Technology
Transition metal selenide films as OER catalysts: The transition metal chalcogenides, which were the main theme of the proposed work, has indeed shown potential for water splitting and the most prominent outcome of this research has been the unexpected discovery of highly efficient oxygen evolution catalysts from the Ni-based selenides which shows much lower onset potential than the precious metal oxides as well as high current density. The highest performing compositions are: Ni3Se2, NiSe2, CoSe, as well as mixed metal selenides, NixFeySez, NixCoySen, and NixAlyFezSen.
The Ni-selenide based films including that of Ni3Se2, NiSe2, NiSe, cobalt selenide, CoSe and mixed metal selenides, NiFe2Se4, Ni2CoSe2, and NiAlFeSe4 were grown through electrodeposition on conducting substrates such as Au-coated glass, Au-coated Si, glassy carbon (GC) as well as Ni foam. Typically the following chemical reactions were carried out for electrodeposition:
mNiSO4 + nNa2SeO3 + LiCl → NixSey [x:y = 1:1 (NiSe); 3:2 (Ni3Se2); and 1:2(NiSe2)] ….(1)
NiSO4 + MxSO4 + Na2SeO3 + LiCl → NixMySen [M = Fe, Co] …(2)
NiSO4 + MIxSO4 + MIIxSO4 + Na2SeO3 + LiCl → NixMIyMIIzSen [MI = Fe], [MII = Al, Co, Mn]…(3)
(pH = 2.5 – 4.5; potential = -0.8 to -1.2 V).
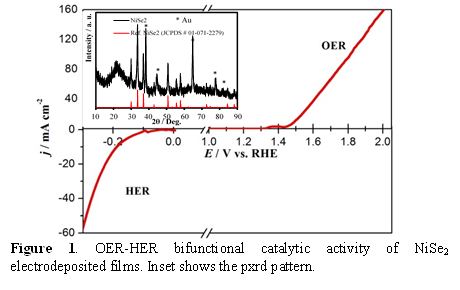
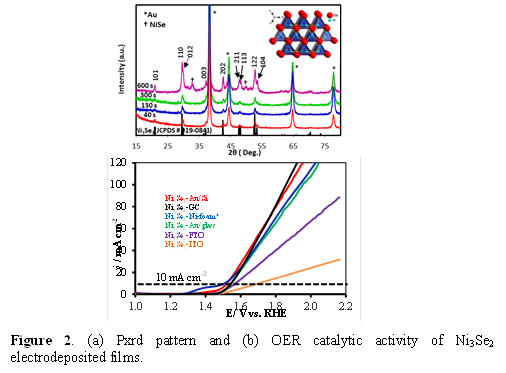
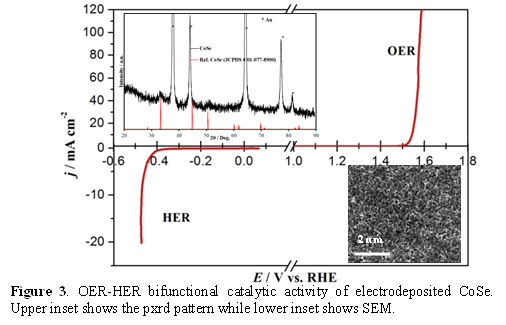
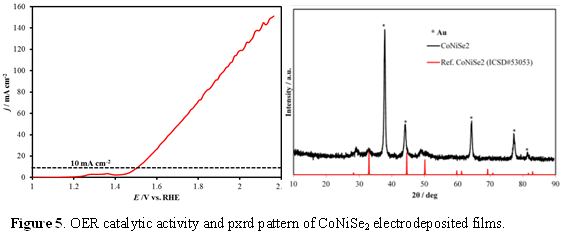
The electrodeposited films exhibited continuous growth on the substrate and showed increasing film thickness as well as increased catalyst loading with increasing deposition time. Catalytic activities of these electrodeposited films were thoroughly investigated through detailed electrochemical studies including linear scan voltammetry (LSV) (Figure 1 - 6), chronoamperometry, simultaneous ORR-OER measurements, Tafel slope analysis, electrochemical surface area measurements and determination of Faradaic efficiency. LSV plots indicated that the onset potential for O2 evolution with these catalysts were lower than most of the commonly used oxides, and some of these selenides even showed a very small overpotential for reaching a current density of 10 mA.cm-2. Among the nickel selenides, NiSe2 exhibited the best catalytic efficiency (overpotential of 210 mV @ 10 mA.cm-2 as shown in Fig. 1) followed by Ni3Se2 (310 mV overpotential @ 10 mA.cm-2, Fig. 2) and NiSe (overpotential of 320 mV @ 10 mA.cm-2). Long term chronoamperometry studies also showed that the catalysts were very stable in alkaline medium under conditions of continuous O2 evolution. The electrodeposited CoSe films exhibited catalytic activity towards OER with a fairly low onset overpotential (220 mV) and low overpotential at 10 mA.cm-2 (310 mV) (Figure 3). Interestingly these films showed low Tafel slope (~33 mV.dec-1) indicating that these O2 evolution process was fast and kinetically favored.
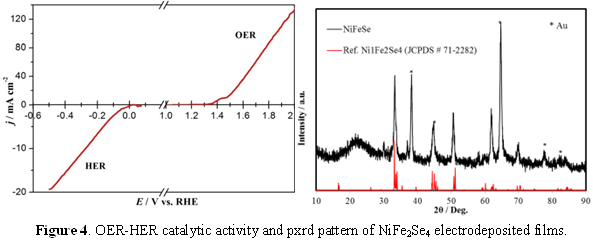
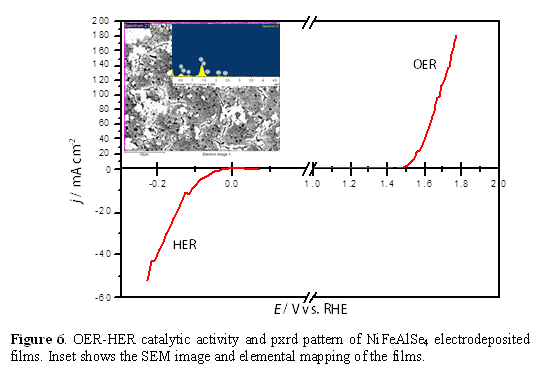
Mixed Metal Chalcogenides as OER Catalysts: Interestingly, for Ni3Se2 we observed that after prolonged O2 evolution in alkaline water, the catalyst showed lower onset potential and higher current density. Compositional analysis of the catalyst post-OER activity revealed that there was some trace amount of Fe incorporation in the catalyst composition which can be linked with the enhanced catalytic activity. This led us to the idea that these Ni-selenides can be doped with Fe to possibly increase the catalyst activity. Thus we intentionally prepared Ni-Fe-Se compositions as well as other mixed metal ternary selenides including NixCoySez, and NixFeyAlzSen systems. These compositions were typically electrodeposited from electrolytic bath containing the metal precursors in varying relative ratios along with sodium selenite or SeO2 in acidic pH (eq. 2). By optimizing the deposition parameters we obtained NiFe2Se4 films which showed enhanced OER catalytic activity with an onset potential of 1.44 V (vs RHE) reaching 20 mA.cm-2 at an overpotential of 210 mV (Figure 4), which again is better than most of the reported oxide catalysts. The other mixed metal selenides also showed much lower onset potential for O2 evolution as well as low overpotential at 10 mA.cm-2 , viz. 280 mV for Ni-Co-Se (Figure 5), and 310 mV for NiFe2-xAlxSe4 (Figure 6).
Transition Metal Selenides and Bifunctional OER-HER Catalysts: While OER activity of the selenides has not been widely researched, NiSe2, and CoSe2 has been predicted to be an effective HER catalyst based on band structure calculations, and indeed has been reported as such.95 Hence, we have tested the HER catalytic activity for these selenides, and observed that the electrodeposited NiSe2 showed high activity for hydrogen evolution as well in alkaline solution (Figure 2). The CoSe films also showed good catalytic activity for hydrogen evolution in the same alkaline solution as OER (Figure 3) where the onset potential for H2 evolution was 0.2 V vs Ag/AgCl, while a current density of 10 mA.cm-2 was reached at an overpotential of 490 mV. While the HER catalytic activity is certainly lesser than Pt, the novelty lies in the fact that these selenides are bifunctional catalysts which is active for both OER and HER in the same alkaline electrolyte. By coating these selenides in both cathode and the anode we could effectively split water into H2 and O2, respectively under ambient conditions by applying a voltage as low as 1.6 – 1.8 V, highlighting the high practical usage of these materials.
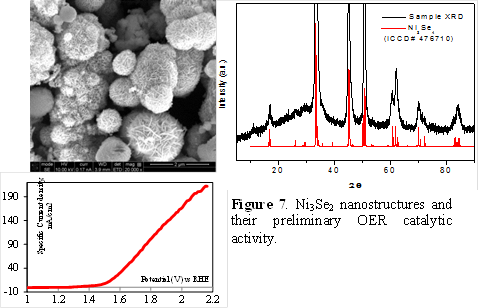
Ni3Se2 nanostructures as OER Catalysts: The authors have hydrothermally synthesized the Ni3Se2 “fuzzy ball”–like structure which is made by aggregation of thin nanoplates of Ni3Se2 (Figure 7). These structures have high surface roughness which can lead to high catalytic activity and preliminary studies showed that these Ni3Se2 fuzzy nanoballs were indeed catalytically active for OER. The authors could grow Ni3Se2 nanoring arrays through patterned electrodeposition on top photoactive CdS layers (Figure 8). The nanoring architecture will be very useful for solar-to-fuel energy conversion, since it has porosity for enhanced catalytic activity, reduces material usage and provides a robust interface at the electrocatalyst-semiconductor junction.
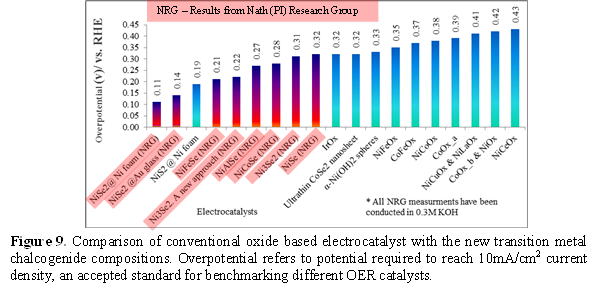
In summary, this project has shown that indeed transition metal chalcogenides has a lot of potential for water oxidation and might have better performance than the oxides (Figure 9).
The PRF funds were mainly used for student and post-doctoral support and some of these results described above has been submitted for publication [Ni3Se2 (Energy Environmental Science, under revision); CoSe (ACS Nano), NiFe2Se4 (Advanced Materials). We have also submitted a NSF proposal in Chemical Catalysis program based on this research and the outcomes.