Reports: DNI554143-DNI5: Assessing the Wettability Alteration of Carbonate Minerals at Nano-Scale
Yuanzhi Tang, Georgia Institute of Technology
The main goal of this research is to elucidate the molecule to nano scale interactive mechanisms between complex organic/inorganic molecules and mineral surfaces, and the impact of such interactions on the surface charge, structure, and reactivity of minerals at nano scale. For the past year, we focused on the interaction between a group of phosphorus containing polyanion with great environmental and biological relevance (polyphosphate) and environmental minerals (iron oxides, aluminum oxides, and carbonates).
Polyphosphates are the polymers of at least three phosphate ions joined by phosphoanhydride (P-O-P) bonds. They are a group of phosphorus (P) species that is widely used as surfactants for many industrial processes, and are also natural molecules that are synthesized by almost all organisms, including a wide range of microorganisms such as bacteria and planktons in both terrestrial and marine settings. They can occur in linear, ring, or branched structures, although the linear structures are the most common form in nature. Polyphosphates can be organic (e.g. ATP and other nucleotide triphosphates), or inorganic. During common cell events, such as extracellular release, lysis, death and burial of microorganisms, polyphosphates are released into water bodies and incorporate into sediments, where they likely undergo a range of transport and transformation processes, such as biotic and abiotic hydrolysis, release of inorganic orthophosphate, as well as the precipitation and formation of phosphate mineral phases. Understanding the fate and transport of polyphosphate in natural and engineered environment is important for determining the impact of polyphosphate-based surfactants. This system is also used as a model system for our study of organic/inorganic polyanion interaction with mineral surfaces and modification of surface reactivities which can occur during various stages of oil reservoir rock diagenesis and oil exploration.
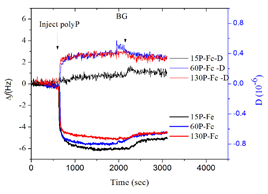
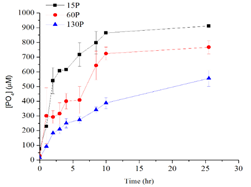
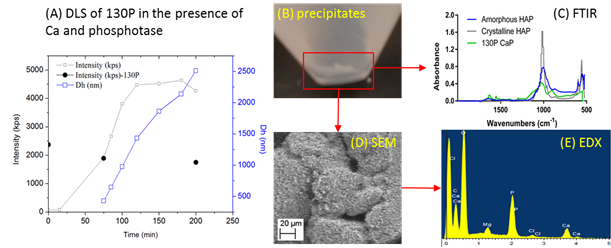
We tested the sorption of polyphosphate with different chain lengths (15, 60, and 130 P units) onto common iron and aluminum oxide surfaces under varied pH conditions and in the presence of common chemicals found in brine (e.g. Ca2+, Mg2+) and microbial metabolites (e.g. phosphatase enzyme). To characterize the reaction mechanisms, kinetics, as well as the impact of dynamic environmental parameters on the interaction mechanisms, we used a suite of complementary nano to molecular scale characterization techniques, such as quartz crystal microbalance with dissipation (QCM-D), dynamic light scattering (DLS), atomic force microscopy (AFM), nuclear magnetic resonance (NMR) spectroscopy, scanning electron microscopy (AFM), and Fourier transformed infrared (FTIR) spectroscopy. We found that the linear polyphosphates were adsorbed onto mineral surfaces with the deprotonated side groups instead of the terminal groups via the formation of inner-sphere complexes with high affinity. As pH increase to 10, there was much less adsorption of the polyphosphate onto both minerals compared to that at lower pH conditions (4, 6 and 8), due to the electrostatic repulsion between the molecules and the surfaces. There was a difference in the rigidness of the adsorbed polyphosphate layer between different minerals and pH conditions, as demonstrated by the dissipation to frequency ratio. The presence of calcium in the solution significantly enhanced the adsorption of polyphosphate, probably due to the bridging of the polyphosphates by calcium. At high calcium concentrations and in the presence of phosphatase enzyme, calcium phosphate precipitates with amorphous nature was found, which is likely to modify the surface structure and properties of the parent mineral. There are ongoing studies based on microcalorimetry and spectroscopy to quantitatively measure the interaction thermodynamics and characterize the speciation at the interfaces. We will further study the effects of surface morphology (flat or curved) and surface impurities on the interaction. We expect the results from this systematic investigation at molecular scale will provide insights to the general mechanisms of polyanion interaction with minerals and modification of mineral surface structure and reactivities within natural and engineered systems.
Figure 1. Quartz crystal microbalance with dissipation (QCM-D) measurements showing the frequency and dissipation change due to polypP sorption onto iron oxide coated sensors at pH 6. Data showing three types of polyP with varied chain lengths (15, 60, and 130P).
Figure 2. Enzymatic hydrolysis of dissolved polyphosphates with different chain lengths (15, 60, 130 P) and the production of orthophosphate in the presence of alkaline phosphatase over time.
Figure 3. Hydrolysis of polyP and precipitation of Ca-P mineral(s) in the presence of phosphatase and Ca2+. (A) Real time observation of solid particle formation using dynamic light scattering, (B) picture of the white precipitates after 24 hr, (C) Fourier transformed infrared (FTIR) spectra of the precipitate revealed similar building units as crystalline and amorphous hydroxylapatite (HAP), (D) and (E) are scanning transmission electron microscopy (SEM) and energy dispersive spectra (EDX) showing the morphology and composition of the precipitate. Ca:P ratio of the precipitate is ~1:1, different from that of HAP (~1.67), suggesting amorphous precursor form(s) of Ca-P mineral(s).