Reports: ND654607-ND6: Non-Equilibrium, Atmospheric-Pressure Microplasmas for Hydrocarbon Gas Conversion
R. Mohan Sankaran, PhD, Case Western Reserve University
Daniel J. Lacks, Case Western Reserve University
1. Introduction
The goal of this ACS PRF ND project is to develop and evaluate an atmospheric-pressure plasma for the non-equilibrium, non-oxidative dissociation of methane and selective conversion to hydrogen, acetylene, and other higher order hydrocarbon gases. In the first year of the project, funds were used to support an undergraduate student, Joseph Toth, and set up the experimental system.
2. Experimental details
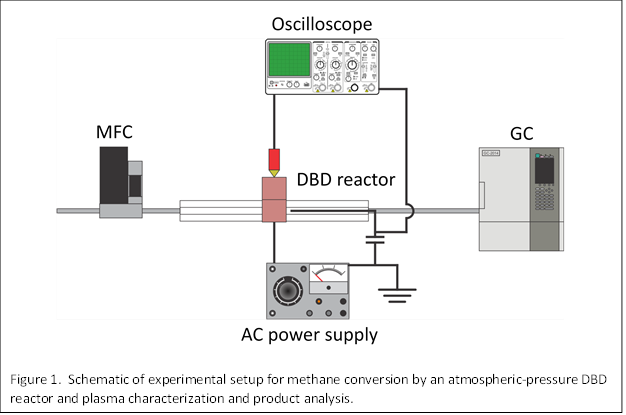
Figure 1 schematically illustrates the atmospheric-pressure dielectric barrier discharge (DBD) reactor and on-line gas chromatography system. Briefly, the DBD was fabricated as a flow-through device with a quartz tube surrounded by a copper strip and a tungsten wire in contact with the gas flow. The DBD was operated by a high-voltage alternating current (AC) power supply. The voltage and current waveforms were monitored by a digital oscilloscope. The volumetric flow rate of methane through the plasma reaction zone was controlled by a mass flow controller (MFC). The product stream was continuously sampled by a gas chromatograph (GC) equipped with thermal conductivity detector (TCD) and flame ionization detectors (FID).
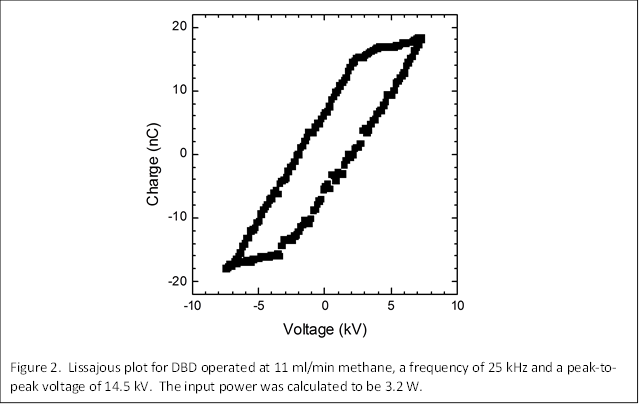
The applied voltage is dissipated in the reactor as displacement current and current absorbed by the gas. To determine the input power, we measured the charge, Q, using a capacitor in series with the DBD (see Figure 1), subtracted the charge due to the displacement current, and constructed Lissajous plots as shown in Figure 2. The area of this plot yields the energy, and dividing by the time (period of the voltage cycle) gives the input power. This analysis was extended to frequencies ranging from 20 to 35 kHz and peak-to-peak voltages ranging from 14 to 18 kV.
Methane conversion was obtained by relating the peak areas from gas chromatograms to molar flow rate. The fractional conversion, f, was defined as:
where ![]() is
the molar flow rate. Based on our analysis which indicated that the change in
total moles due to reaction was negligible, we assumed constant density. Product
selectivity was similarly calculated from the peak areas measured by GC and
calibrating with a reference gas mixture. The selectivity of a species, Si,
was defined as:
is
the molar flow rate. Based on our analysis which indicated that the change in
total moles due to reaction was negligible, we assumed constant density. Product
selectivity was similarly calculated from the peak areas measured by GC and
calibrating with a reference gas mixture. The selectivity of a species, Si,
was defined as:
We note that two products, hydrogen and solid carbon, required special methods of characterization. Hydrogen was measured by the TCD, while all other hydrocarbons were measured by the FID, which in general has better signal sensitivity but cannot detect hydrogen. The amount of solid carbon, which was primarily deposited on the reactor walls and could not easily be collected, was estimated from independent mass balances on carbon and hydrogen which showed good correspondence.
3. Results
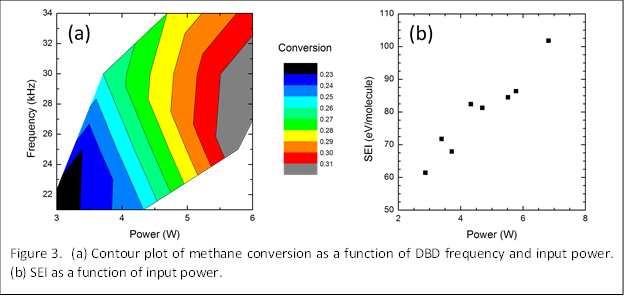
Figure 3a shows a summary of our results for methane conversion in the DBD reactor as a function of frequency and input power. We note that the input power is related to the frequency and therefore these trends are not completely independent. However, power is a stronger function of applied voltage than frequency and, thus, the frequency dependence does show the effect of other properties of the plasma. The results show that methane conversion increases with frequency and power. The plasma density is known to increase with power, which in this case is proportional to methane dissociation. The increase in conversion with frequency is more complicated, but we suggest that electron heating may be enhanced at higher frequencies, leading to more energy available to dissociate methane molecules.
We evaluated the energy efficiency of our reactor for methane conversion by calculating the standard energy input (SEI), the energy input per molecule converted. SEI was defined as:
where P is the input power. Figure 3b shows the SEI as a function of input power at the various conditions studied thus far. The SEI is found to increase with power which indicates that although methane conversion is improved at higher powers, the energy efficiency decreases.
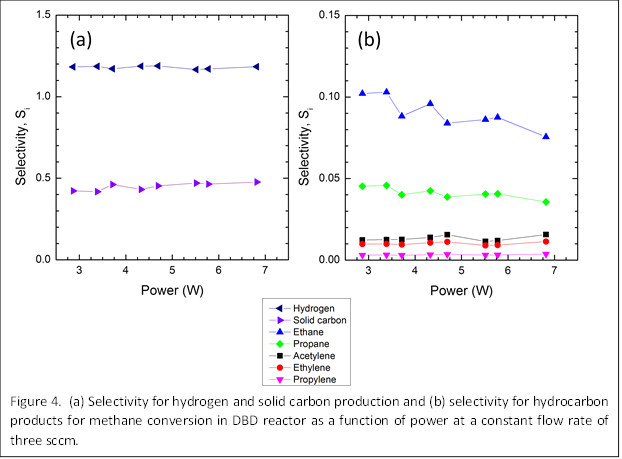
We have also obtained preliminary results for the product selectivity. As should be expected, hydrogen and solid carbon make up the majority of the products (Figure 4a). Among the hydrocarbon products, ethane was found to have the highest selectivity, followed by propane, acetylene, ethylene, and propylene (Figure 4b). The selectivities were not found to significantly change with input power. The high selectivity to ethane suggests that the dominant mechanism for methane conversion in our process is dissociation to methyl radicals (CH3) and simple recombination. In comparison, there may be very little production of methylene (CH2) and methylidyne (CH) radicals that would lead to the formation of ethylene, acetylene, and propylene. We are now carrying out additional experiments and modeling to support these general mechanisms and clarify the details.
4. Impact of project
This project has established a new direction of research for the PI, Prof. Sankaran, and Co-PI, Prof. Lacks in the area of gas conversion by plasma-based approaches. We are learning techniques related to methane dissociation and product formation. The project has also exposed the undergraduate student, Joe, to research on this topic and motivated him to continue onto graduate school. He is now pursing his Ph.D. under the supervision of Prof. Sankaran and Prof. Lacks.