Reports: DNI454396-DNI4: Probing Spin-Exciton and Spin-Charge Interactions in Open-Shell Organic Semiconductors
Trisha L. Andrew, University of Wisconsin-Madison
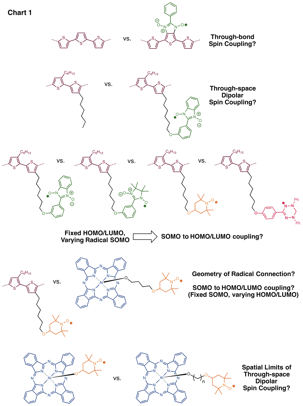
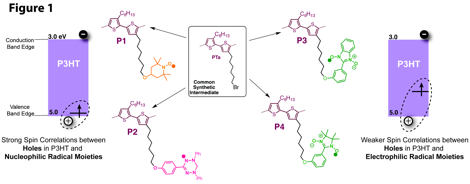
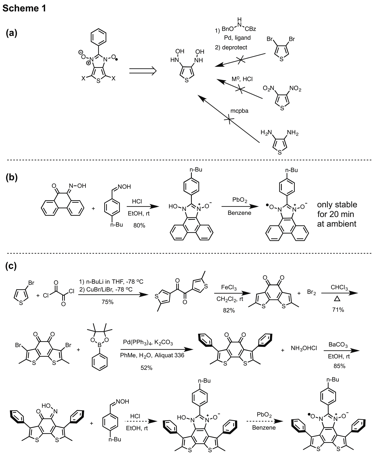
Trisha L. Andrew, University of Wisconsin-Madison



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society