Reports: UNI353723-UNI3: Chelation-Assisted Au(III) Functionalization of Strong, sp3-Hybridized C-H Bonds, Similar to Those Found in Alkanes
David R. Weinberg, PHD, Colorado Mesa University
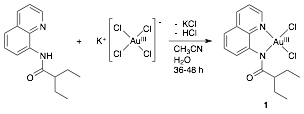
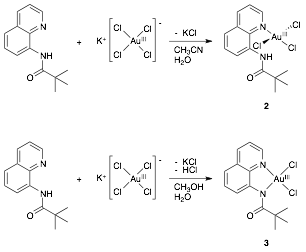
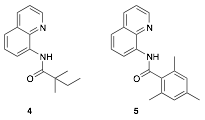
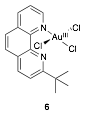
David R. Weinberg, PHD, Colorado Mesa University




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society