Reports: UNI453797-UNI4: Investigating the Mechanism of Aryl-Alkyne Cyclization of Azaborine Containing Aromatics
Eric H. Fort, PhD, University of St. Thomas
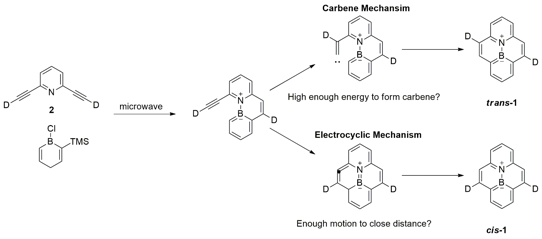
Our grant aims to understand the mechanisms of aryl-alkyne ring-closure for molecules containing boron-nitrogen (azaborine) bonds. In previous work attempting to improve the synthesis of BN-pyrene (1, Scheme 1), we found that we could induce an alkyne cyclization at relatively low temperatures without the presence of a catalyst. This type of transformation is highly endothermic in the parent hydrocarbons. It was proposed that either low temperature carbene formation or electrocyclic closure could lead to improved routes to a number of azaborine bond containing polycyclic aromatic molecules. The mechanism had not been studied with azaborine molecules, and the low-temperature effect may be unique to these systems. Through isotopic labeling and computational studies, we are working to understand this mechanism and use it in the design and synthesis of new compounds.
Scheme 1: Isotopic labelling of 2,6-diethynylpyridine can help decipher the mechanism of aryl-alkyne ring closure in the production of BN-pyrene (1).
During the first year of our funding we focused on three main areas (1) developing an efficient method for isotopic labelling for our mechanistic study, (2) calculating the energetic barriers involved in the aryl-alkyne ring closure, and (3) synthesizing the isotopically labeled BN-pyrene to confirm our mechanistic hypothesis. We have been successful in all three of these goals and the results have given clear priority for the current funding year as will be described below.
(1) Developing an efficient method for Isotopic labelling:
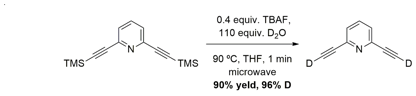
In order to perform our mechanistic study, an efficient method for incorporating deuterium onto the terminus of 2,6-diethynylpyridine (2, Scheme 1) was required. Traditional methodologies required producing the protonated version, deprotonating with strong bases, and quenching with deuterium. We envisioned a milder system that could in one-pot desilylate the Sonagashira products and directly install deuterium. After a great deal of optimization we were able to develop a system that efficiently preforms this task in one minute to give greater than 90% yield and up to 97% deuterium incorporation (Scheme 2). We demonstrated this with four different ethynylpyridine compounds and published our work in Tetrahedron Letters in the spring of 2015.
Scheme 2: Optimized reaction conditions for a mild, one-pot, desilylation and deuteration of trimethylsilyl-protected alkynylpyridines.
(2) Calculating the energetic barriers or aryl-alkyne ring closure:
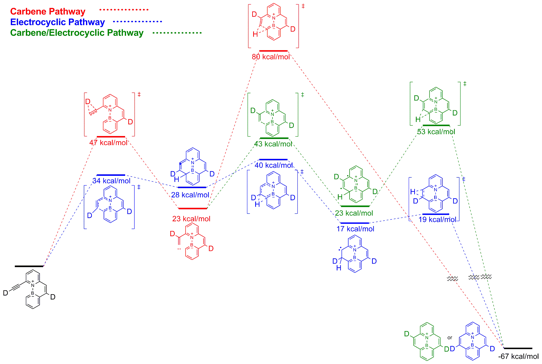
We turned to computation to support our proposed mechanisms of closure. Using the computational chemistry program Spartan, we were able to model both the electrocyclic and carbene closure mechanisms as well as a hybrid closure where the carbene is formed and the closure is the result of an electrocyclic cascade (Figure 1). We found three important results. First, the calculations predict a purely electrocyclic pathway to be the lowest energy route for closure. Second, the activation energy for the azaborine containing molecule is dramatically lower than the hydrocarbon analog. Third, the predicted mechanism of closure is different than that of ethynylbiphenyl systems that have previously been calculated. As a result of these findings, we have set about calculating the mechanism for the hydrocarbon ethynylphenanthrene system to see whether it is the azaborine or the larger pi-system causing this effect. We have also started synthesizing ethynylphenanthrene as a control experiment for our mechanistic study. These actions will constitute a portion of our goals for the coming year.
Figure 1: Computational study of the aryl-alkyne ring closure showing that the electrocyclic pathway (shown in blue) is favored in the production of BN-Pyrene. (B3LYP/6-31G**)
(3) Synthesis of BN-Pyrene-d2
With highly enriched 2,6-diethynylpyridine-d2 in hand, we set about conducting the mechanistic study for the production of BN-pyrene. The groundwork for the ultimate reaction in this synthetic sequence is not trivial, and it took a greater portion of the year to produce the starting materials in great enough quantity and high enough purity for the mechanistic study. We attempted the synthesis several times and were able to observe deuterated product in the crude NMR spectra. In the absence of catalyst and at the high temperatures for conversion to product, a great deal of material is lost to polymerization and oxidation of the precursors. As such isolated yields were not reliable. However, multiple experiments resulted in the same conclusion: Any material able to cyclize to BN-pyrene produced the cis-deuterated product in nearly the same isotopic incorporation as the starting deuterated 2,6-diethynylpyridine. If 2,6-diethynylpyridine with 80% deuterium labeling was put into the reaction, the crude NMR would identify BN-pyrene-d2 with approximately 80% deuterium incorporation in the cis-arrangement (Figure 2). This experimentally confirms our computations that the electrocyclic closure is the preferred mechanism for BN-pyrene closure.
Figure 2: Synthesis of BN-Pyrene-d2 gives evidence of the electrocyclic closure as the preferred mechanism, in concordance with the computational results.
Impact and Future Work:
This work supported three students and over the past year, with one graduating in the spring of 2015 and starting his graduate career in chemistry at the University of Minnesota. Two of the three students were able to publish their work this past year and all have presented at local and national conferences. The two remaining students continue to work on our control experiment to support the significance of the azaborine bond in lowering the energy of cyclization as we prepare these results for publication. Both of these students have showed interest in attending graduate school in the future. More broadly, these results have already inspired the design of new molecules, and we pursue them presently.