Reports: DNI151710-DNI1: Enantioselective Allylic Amination of Olefins
Uttam K. Tambar, PHD, University of Texas Southwestern Medical Center at Dallas
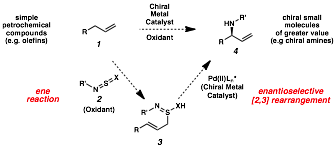


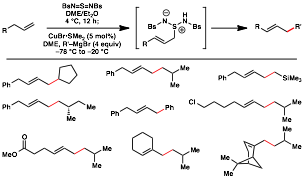
Uttam K. Tambar, PHD, University of Texas Southwestern Medical Center at Dallas




Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society