Reports: UR352779-UR3: Triazole-Containing Tridentate Anionic Chelators for Lanthanide and Group VIII Organometallic Complexes
James T. Fletcher, PhD, Creighton University
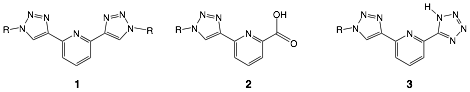
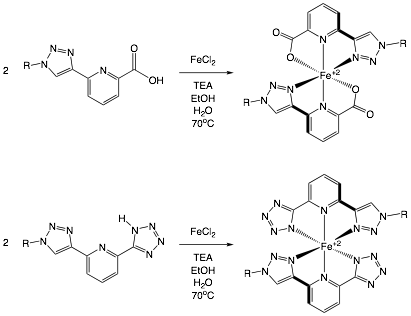
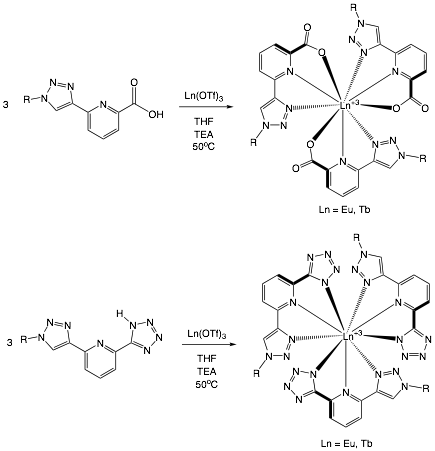
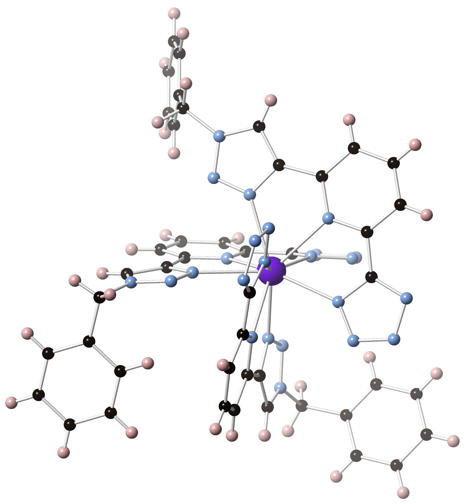
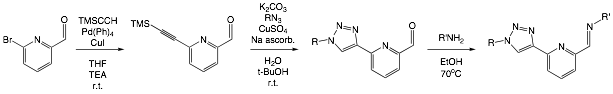
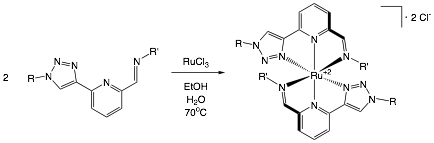
James T. Fletcher, PhD, Creighton University






Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society