Reports: DNI653049-DNI6: Characterization of Micelle Formation and Stability Under Extreme Conditions
Silvina Matysiak, PhD, University of Maryland
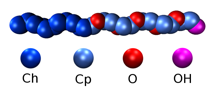
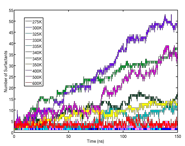
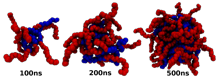
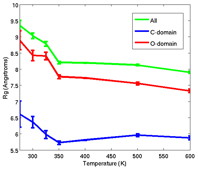
Silvina Matysiak, PhD, University of Maryland





Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society