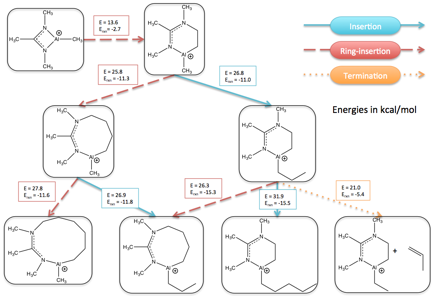
Reports: DNI654267-DNI6: Characterization and Screening of Al-based Olefin Polymerization Catalysts Using Automated Reaction Discovery Approaches
Paul Zimmerman, PhD, University of Michigan
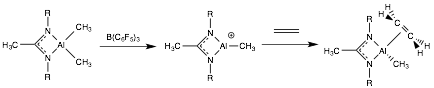
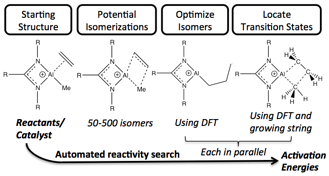
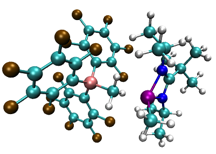
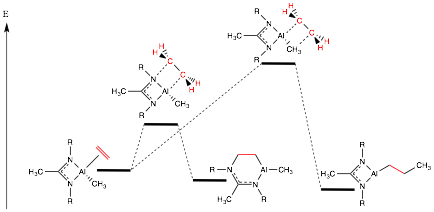
Paul Zimmerman, PhD, University of Michigan





Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society