Reports: DNI753461-DNI7: Phase Behavior of Giant Spherical Amphiphilic Block Copolymers in Solutions
Zhihong Nie, PhD, University of Maryland
Micelle architectures have emerged as a remarkable class of building blocks for generating hierarchically structured functional materials. Although molecular self-assembly is well-studied, much less is known about the self-assembly of micelle building blocks. The scientific objective of this research program is to explore the self-assembly and phase behavior of spherical micelle architectures in selective solvents by using a model system. Our rationale is that inorganic nanoparticles (NPs) grafted with multiple amphiphilic block copolymers (BCPs) (referred to as giant spherical amphiphilic BCPs, GSA-BCPs) can serve as a good model of micelle architectures by mimicking their physical and chemical traits.
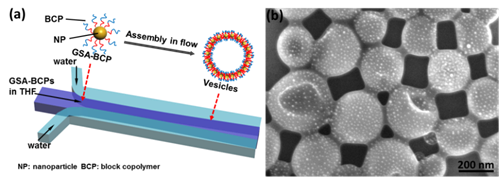
Figure 1. (a) Schematic illustration of the self-assembly of GSA-BCPs into vesicles in microfluidics. (b) Representative SEM image of vesicles assembled from GSA-BCPs containing 5-nm AuNP core.
First, we have created a model GSA-BCP that allows us to systematically explore the role of thermodynamic and kinetic parameters of the self-assembly of micelle architectures in selective solvents. In our paper published in ACS Materials and Interfaces, we reported the effect of hydrodynamic flow on the assembly of Au NP (AuNP)-based GSA-BCPs into vesicles with a monolayer of GSA-BCPs in the membranes using laminar flows in microfluidic devices (Figure 1).
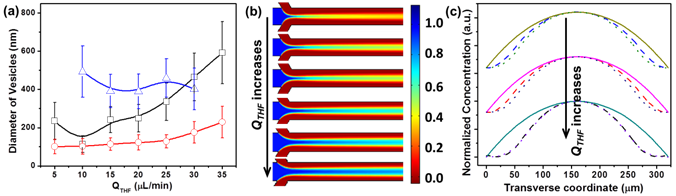
Figure 2. (a) The diameter of vesicles assembled from pure BCPs (red), GSA-BCPs with 5 nm-AuNP core (black) and 20 nm-Au core (blue) as a function of the flow rate of building blocks in tetrahydrofuran (THF) (QTHF). (b) Simulated GSA-BCPs concentration distribution at various QTHF. (c) Simulated concentration profiles of pure BCPs (solid line), GSA-BCPs with 5 nm-AuNP core (dash line) and 20 nm-AuNP core (dot line) as a function of the transverse coordinate of the microchannel.
Our experimental and simulation results suggest that the competitive diffusion of solvent/GSA-BCPs along the transverse direction of the microchannel plays an important role in the assembly process (Figure 2). The diffusion coefficient of a species (i.e., solvent molecules and GSA-BCPs) is inversely proportional to their size. For molecular amphiphiles, their size and diffusion rate are generally on the same order of magnitude as the solvent molecules. In this case, there is no clear boundary of the concentration gradient of solvents and amphiphiles. In contrast, the significant increase in the dimension of GSA-BCPs drastically decreases their diffusion rate. The slower transverse diffusion of GSA-BCPs leads to a significant difference in the gradients of solvents and GSA-BCPs (Figure 2c). This phenomenon strongly affects the kinetic process of GSA-BCP self-assembly, as reflected by the big difference in the dimension of assembled structures from GSA-BCPs and conventional BCPs (Figure 2a).
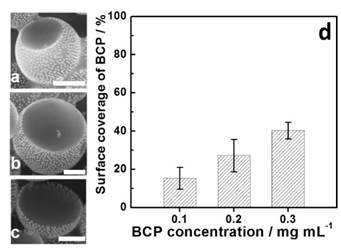
Figure 3. (a-c) SEM images of Janus-like vesicles assembled from a mixture of GSA-BCPs and free BCPs with different BCP contents. The scale bars are 500 nm. (d) The percentage of AuNPs-rich domain as a function of free BCP concentration.
Second, we have exploited the phase separation behaviors of a mixture of GSA-BCPs and free (unbonded) BCPs in selective solvents using laminar flow in a microfluidic device. In our paper published in Small, we reported that the assembly of a mixture of amphiphilic BCPs and GSA-BCPs generates Janus-like hollow vesicles with two distinct halves: polymer-rich half and NP-rich half. The relative surface ratio of polymer-rich domain to NP-rich domain in the Janus vesicles can be tuned by controlling the ratio of individual building blocks in the mixture. With the increase of free BCP concentration from 0.1 to 0.3 mg/ml, the percentage of polymer-rich domain coverage increased from 15.4% to 40.4% (Figure 3).
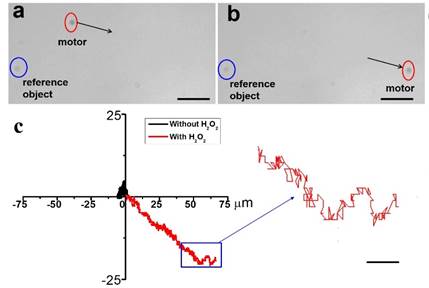
Figure 4. Motion of Janus vesicles containing PtNPs as catalytic motors. (a, b) The snapshots of vesicular motor (red circle) movement in the presence of 10% hydrogen peroxide. (c) The trajectory of vesicular motor with (black) and without (red) the addition of hydrogen peroxide as chemical fuel. Scale bars are 20 µm (a,b) and 4 µm (c).
When platinum NPs (PtNPs) are used as the core of GSA-BCPs, the Janus vesicles can undergo autonomous propulsion through the decomposition of hydrogen peroxide (Figure 4). In the absence of fuel, the vesicle showed a typical random walk behavior as a result of Brownian motion. The trajectory of motion was random and localized within a small area. When H2O2 was added, the motion of the catalytic vesicular motor was relatively directional. The motion velocity of the motor can be controlled by adjusting the concentration of H2O2. The average velocity of the Janus motor increased with the increase of H2O2 concentration, as a result of faster catalytic decomposition of H2O2 at higher concentration.
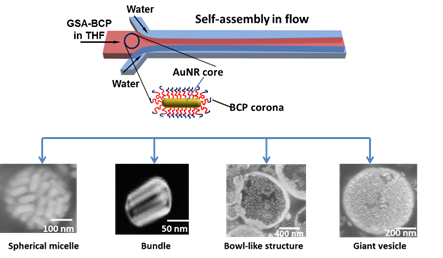
Figure 5. The kinetic control over the self-assembly of GSA-BCPs comprising Au nanorod (AuNR) core. Assemblies including spherical micelle, bundle, bowl-like vesicles, and giant vesicles are formed at different hydrodynamic conditions.
Finally, we have studied the kinetic aspects of the self-assembly of GSA-BCPs comprising Au nanorod (AuNR) core in a microfluidic device, as well as the shape effect of GSA-BCPs on the assembly morphology. We are currently preparing a manuscript on our findings of assembly kinetics. By varying the flow rates of each fluid, we precisely control the diffusive mixing of the miscible solvents and hence the kinetic pathways of GSA-BCP self-assembly. Depending on the hydrodynamic conditions, the assembly process produced assemblies with different morphologies, including bundles, micelles, bowl-like structures (i.e., vesicles with openings), and vesicles. In contrast to the formation of vesicles with openings for rod-like GS-BCPs at certain hydrodynamic condition, small spherical GSA-BCPs prefer to assemble into vesicles without defects.
The research program involves three postdoctoral researchers, two graduate students (Yijing Liu and Shaoyi Zhang), one undergraduate student (Yunlong Yang), and one assistant professor. The results generated in this work constituent a significant portion of the PhD thesis of Yijing Liu who has just graduated in the summer of 2015. The project has led to two publications and one manuscript in preparation. The undergraduate student is one of the co-authors of the paper in Small. Moreover, the knowledge obtained from this work leads to us to the development of excitingly new research directions, such as on the design and assembly of supracolloidal molecules.