Reports: DNI554216-DNI5: Ethene-to-Propene Metathesis on Nickel-Exchanged Zeolites: A Fundamental Kinetic Study of the Effects of Pore Size and Nickel Structure on Ethene Dimerization Rates
Rajamani Gounder, PhD, Purdue University
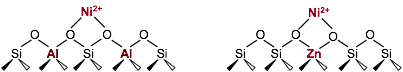
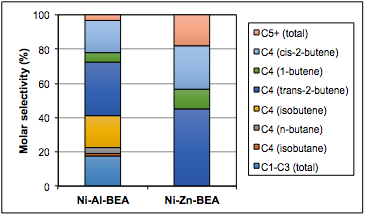
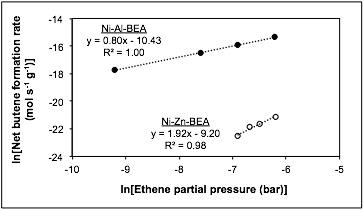
Rajamani Gounder, PhD, Purdue University



Reports in the ACS PRF Annual Report are published as submitted by the Principal Investigator.
Copyright © American Chemical Society